Fig. 9.1
Comparison of prostate cancer incidence rates globally. Rates are age-adjusted for comparisons across countries and are presented per 100,000 in the population. Globocan 2012
Age-adjusted incidence rates have increased in notable patterns over time across the world (Fig. 9.2), particularly in the United States, Europe, and Australia, paralleling the uptake of PSA screening. However, incidence rates have also increased in Japan and some other Asian and Eastern European countries where PSA testing has to date not been widely used [13]. PSA screening has also led to a shift in stage presentation, with an increased proportion of localized prostate cancer disease as well as an earlier age at diagnosis [14]. Another consequence has been the substantial overdiagnosis of prostate cancer, i.e. the detection of a significant number of cancers that may never have come to light clinically nor harmed a man during his lifetime [15, 16].
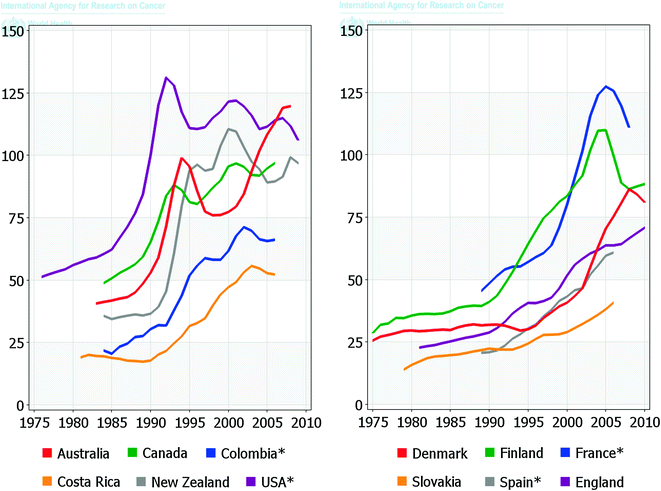

Fig. 9.2
Trends in age-adjusted prostate cancer incidence rates over time in selected populations. Rates are presented per 100,000 in the population. Globocan 2012
Additionally, PSA screening has likely been changes in the observed associations between specific lifestyle factors and risk of total prostate cancer in epidemiological studies. First, lifestyle factors may impact prostate cancer at various stages from initiation to progression to metastases. As such, the associations may differ according to disease clinical characteristics, such as those defined by cancer stage or tumor grade [17]. Indeed, it seems unlikely that the factors associated with development of indolent cancers would be similar to those associated with cancers demonstrating malignant potential. Second, PSA screening is a strong potential confounder, as screening behaviors tend to be associated with other healthy behaviors as well as strongly associated with prostate cancer incidence. Thus, an assessment of the quality of epidemiological studies in prostate cancer should include an evaluation of the ability of the study to integrate information on PSA screening.
9.2.3 Mortality
An estimated 307,000 men died of prostate cancer worldwide [9], with a 10-fold variation in mortality rates among countries (Fig. 9.3). The highest prostate cancer mortality rates are among men in Caribbean countries as well as parts of Africa. Prostate cancer is the second most common cause of cancer death among men in the United States, with 26,120 cancer deaths expected in 2016 [10]. Over the past decade, prostate cancer mortality rates have shown declines in many westernized countries. The reasons for this decline remain controversial, but may be attributable in part to earlier detection of prostate cancer through PSA screening and subsequent earlier treatment [18]. In contrast, mortality rates from prostate cancer are rising in countries throughout Africa.
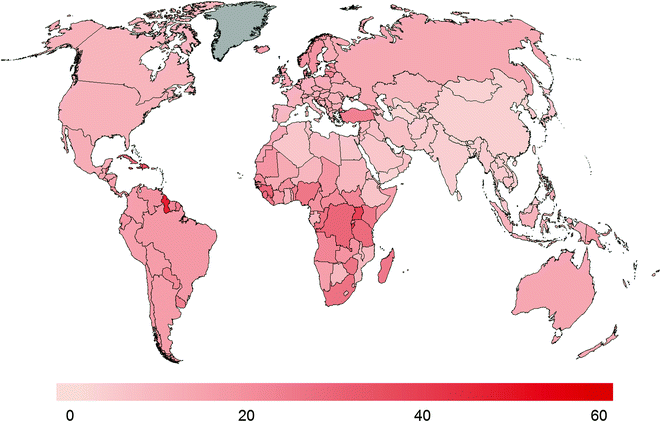

Fig. 9.3
Comparison of prostate cancer mortality rates globally. Rates are age-adjusted for comparisons across countries and are presented per 100,000 in the population. Globocan 2012
Mortality rates are estimated as the number of cancer deaths per 100,000 in the population, and these rates are influenced as a function of both the incidence of the disease and survival among prostate cancer patients. The ratio of incidence to mortality rates range from 10:1 in North America and Australia to 2:1 in Central America and Caribbean to 1.2:1 in parts of Africa. Part of these differences can be attributed on the one hand to the slow growing cancers diagnosed as a result of PSA screening [19, 20] and on the other due to later presentation of disease in countries with little diagnostic intensity.
More than 4 million men are prostate cancer survivors living with a cancer diagnosis around the world, of whom 2.7 million are in the United States [10].
9.3 Risk Factors
9.3.1 Introduction
The prevention of prostate cancer has the potential to improve health and reduce suffering from this common disease. The disease is heterogeneous in its biological potential, and this heterogeneity is an important feature of the disease. While some men have an aggressive form of prostate cancer, most others have a slow growing or indolent form of disease, and risk factors for total versus aggressive prostate cancer may differ. Below, we discuss the evidence surrounding specific lifestyle and dietary factors as potential risk factors for prostate cancer overall as well as for cancers with a lethal potential.
9.3.2 Risk Factors for Total Prostate Cancer
There are few established risk factors for the incidence of total prostate cancer: older age, African–American race, and positive family history. Moreover, there are now more than 105 genetic risk loci that have been identified and confirmed in genome wide association studies [21, 22] in ethnically diverse populations. Taller height is also a probable risk factor for total prostate cancer [23]. It is important to note that none of these factors are modifiable.
Older age is one of the strongest risk factors for prostate cancer. Prostate cancer rarely is diagnosed among men before the age of 40 years. As with other epithelial cancers, the incidence rates of prostate cancer increase exponentially from around age 55 years, a pattern observed across multiple populations. PSA screening diagnoses cancers 10-years earlier than through symptomatic disease (lead-time), and thus widespread screening has led to a shift to an earlier average age of cancer diagnosis. The median age at diagnosis among US men is 66 years.
Prostate cancer incidence and mortality rates differ substantially by race/ethnicity. In the US, incidence and mortality rates are highest among black men (Fig. 9.4), with mortality rates that are 2.4 times greater than among white men. The reasons for the disparity in prostate cancer rates among black men are unknown, although there is some data to suggest differences in access to care and stage at diagnosis may in part explain the differences in prostate cancer mortality [24]. Both incidence and mortality rates are lower among Asian/Pacific-Islanders, Native Americans, and Hispanic men than among non-Hispanic whites [10].
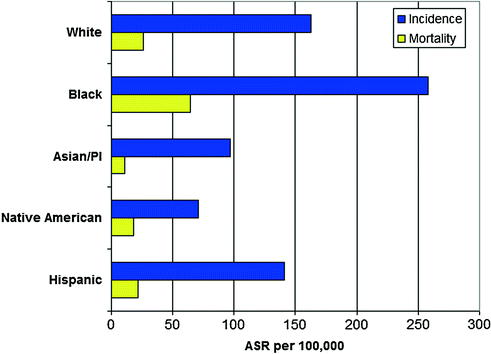

Fig. 9.4
Incidence and mortality of prostate cancer by race/ethnicity in the United States. Rates are age-standardized for comparisons and presented per 100,000 in the population. SEER Registry data
Data from family and twin studies provide strong evidence of a role of family history in overall prostate cancer risk. Men whose father or brother is diagnosed with prostate cancer have a 2–3-fold higher risk than men without a family history. For men with a positive family history in both the father and a brother, the risk increases almost 9-fold [25]. Family history has also been associated with an increased risk of lethal prostate cancer. The risk of death from prostate cancer is approximately two-fold higher for men with a father or a brother who died of prostate cancer compared to men with prostate cancer who do not have a positive family history [26].
The familial aggregation of prostate cancer incidence is in large part due to genetic factors [27] with an estimated heritability from twin studies of 56 % [28]. Multiple genome-wide association studies have been conducted to identify common single nucleotide polymorphisms (SNPs) associated with prostate cancer incidence [29]. To date, more than 105 prostate cancer risk loci have been confirmed across multiple studies [21, 30], and these loci explain about one-third of the heritability. Most of the identified germline risk loci do not appear to be more strongly associated with lethal or nonlethal prostate cancer [31, 32], suggesting that inherited factors may play a role quite early in the pathogenesis of the disease. There are notable differences in the prevalence of several of the genetic risk loci by race/ethnicity men [33], which could account for at least part of the difference in incidence rates.
9.3.3 Risk Factors for Advanced Prostate Cancer
9.3.3.1 Obesity and Weight Change
The obesity epidemic looms large globally, with 1.5 billion adults estimated in 2008 to be overweight or obese [34]. In the US, one-third of adults were obese defined as having a body mass index (BMI) ≥30.0 kg/m2 [35]. Obesity dysregulates multiple hormonal pathways, including higher levels of insulin, lower levels of adiponectin, lower levels of testosterone and sex hormone binding globulin, higher estradiol and higher levels of inflammatory cytokines [36–39].
The relation between body size and incidence of prostate cancer is complex [17, 23, 36, 40–42]. Obese men are at higher risk of developing advanced stage prostate cancer and have higher rates of recurrence and cancer-specific mortality after diagnosis [33, 42]. A meta-analysis of 6 cohort studies found that among men with prostate cancer, a 5 kg/m2 increase in BMI was associated with a 20 % (95 % CI: 0.99–1.46) increased risk of prostate cancer-specific mortality [42]. The association between obesity and poor prostate cancer outcomes do not appear to reflect solely differences in screening, as similar associations are seen after adjusting for stage and grade at diagnosis.
Higher pre-diagnosis levels of C-peptide, a circulating marker of insulin secretion, were associated with increased cancer-specific mortality, independent of BMI [36]. Men who were both overweight and who had high insulin levels had a 4-fold greater risk of death. However, two prospective studies found no association between pre-diagnosis C-peptide and risk of aggressive or advanced disease [36, 43]. Understanding drivers of the association with obesity are critical to understand mechanisms and guide prevention.
Abdominal obesity, as measured by waist circumference, may indicate a more metabolically active obesity. In the European Prospective Investigation into Cancer and Nutrition (EPIC) of 150,000 European men, waist circumference was positively associated with risk of advanced prostate cancer with a 1.06 times greater risk with a 5 cm increase in circumference [44]. Waist circumference was also significantly associated with more aggressive disease in the Melbourne Collaborative Cohort Study [45], but not in the Health Professionals Follow-up Study (HPFS) [46].
Several cohort studies have examined adult weight change and the risk of prostate cancer. Overall, weight gain from early adulthood (age 18 or 21) to mid-life was not associated with prostate cancer incidence in all [46–54] but one study [55]. Only one study has examined weight change in the period shortly before and after prostate cancer diagnosis and the risk of recurrence, measured by post-treatment PSA increase [56]. This retrospective cohort study found that weight gain from five years before treatment by prostatectomy to one year after treatment was associated with statistically significant increase in recurrence, while weight loss was non-statistically significantly associated with lower risk of recurrence.
9.3.3.2 Physical Activity
Physical activity has not been associated with overall prostate cancer risk. However, several studies report an inverse association between recreational physical activity and the risk of advanced prostate cancer. The HPFS [57] and the Cancer Prevention Study (CPS) II [58] studies both reported lower risks of more advanced disease with increasing physical activity. In the CPS II, men reporting the greatest physical activity per week had a relative risk for aggressive cancer (high stage or grade) of 0.69 (95 % CI: 0.52–0.92). The EPIC cohort found no association between recreational physical activity and advanced or high-stage disease; however, activity levels were substantially higher in this cohort, and the reference group included men with up to 25 MET-hours per week.
Among 2705 men with prostate cancer, those who exercised vigorously for 3 or more hours per week had a 61 % lower risk of prostate cancer-specific mortality than those with less than one hour per week of vigorous activity (RR 0.4, 95 % CI: 0.2–0.8) [59]. Both vigorous and non-vigorous activities were associated with lower risk of all-cause mortality among these men with prostate cancer. Similarly, brisk walking was associated with a lower risk of recurrence (RR 0.4, 95 % CI: 0.2–0.9) for those walking 3 or more hours per week versus easy walking for less than 3 h per week [60].
9.3.3.3 Smoking
As with other factors, smoking is not associated with total prostate cancer incidence. However, the latest review of evidence by the United States Surgeon General concluded that smoking is a “probable” risk factor for prostate cancer mortality [61]. In HPFS, greater pack-years of smoking in the 10 years prior to prostate cancer diagnosis were associated with an increased risk of lethal disease, whereas total lifetime smoking was not associated with risk [62]. However, current smokers report less PSA testing than non-smokers [63], and the positive associations between smoking and prostate cancer mortality may be due in part to later diagnosis and treatment of these cancers among smokers.
Smoking may also influence cancer-specific outcomes by influencing response to treatment. Studies in specific treatment populations have consistently reported worse outcomes for smokers than non-smokers among prostate cancer patients treated with radiation, ADT, and radical prostatectomy [64–68].
To date, one prospective study of smoking and cancer-specific mortality among men with prostate cancer has been published [69]. Among 5366 men diagnosed with prostate cancer in the HPFS, there were 524 prostate cancer deaths. The relative risk of prostate cancer-specific mortality was 60 % higher (95 % CI: 1.1–2.3) among current versus never smokers after adjusting for potential confounders. The relative risk was attenuated, although still elevated, when models were further adjusted for stage and grade, which may suggest that part of the relationship between smoking and prostate cancer mortality is through its influence on these clinical parameters. The increased risk of prostate cancer-specific mortality was restricted to men diagnosed with localized or locally advanced cancer (stage T1–T3). Former smokers who quit 10 or more years before diagnosis or who had smoked less than 20 pack-years had the same risk as never smokers.
The possible biological basis for an association between smoking and risk of advanced prostate cancer or survival among men with prostate cancer is not clear, but several mechanisms have been proposed [69]. Tumor promotion through carcinogens from tobacco smoke is a possibility, with several studies finding prostate-cancer specific mechanisms in animal and in vitro studies.
9.3.3.4 Antioxidants
Several dietary antioxidants, including selenium, Vitamin E, and lycopene/tomato sauce have been investigated with respect to prostate cancer incidence. Antioxidants are compounds that inhibit the oxidation of other species, thereby limiting the damaging effects of oxidation in animal tissues. Oxidative stress may damage molecules including proteins and DNA, and has been implicated in carcinogenesis.
Vitamin E and Selenium. Vitamin E generally refers to a group of fat-soluble compounds that include tocopherols and tocotrienols. Alpha-tocopherol is the biologically most active form, and current US dietary recommendations are based on alpha-tocopherol alone. Possible anti-carcinogenic actions of vitamin E include its ability to reduce DNA damage and inhibit malignant cellular transformation [70, 71]. In experimental models, derivatives of vitamin E inhibit growth, induce apoptosis [72] and enhance therapeutic effects in human prostate cancer cells [73].
Secondary results of the Alpha-Tocopherol Beta-Carotene Cancer Prevention (ATBC) Study [74] showed a 32 % reduction in prostate cancer risk among men assigned to alpha-tocopherol supplementation compared to placebo [75]. Another trial of a variety of nutrients found that vitamin E (in combination with selenium and beta-carotene) reduced overall cancer mortality [76]. These results, along with laboratory evidence and some epidemiologic support, motivated two trials of vitamin E supplementation on the risk of prostate cancer.
The Selenium and Vitamin E Cancer Prevention Trial (SELECT) primary prevention study of 50,000 men, planned for 7–12 years, was stopped early because of lack of efficacy for risk reduction. The initial report based on an average of 5.5 years of treatment, found a non-significant suggestion of increased prostate cancer risk among men receiving 400 IU/day of alpha-tocopherol [77]. With additional follow-up the vitamin E group was found to have a statistically significant increase in prostate cancer risk (RR 1.17, 99 % CI: 1.00–1.36, P = 0.008, among 1149 cases) [78]. Interestingly, there was no statistically significant increased risk of prostate cancer in the vitamin E and selenium combination group, suggesting the two may interact. The Physicians Health Study II (PHS II), conducted contemporaneously with SELECT, found no effect on the incidence of prostate cancer (RR 0.97, 95 % CI: 0.85–1.09), with a dose of 400 IU/day for a median of 8 years of follow-up [79].
All men in the ATBC trial were smokers, and the cancers were diagnosed outside the context of PSA screening, and were generally aggressive. In the VITamins And Lifestyle (VITAL) study, a cohort study specifically designed to examine supplement use and future cancer risk, a 10-year average intake of supplemental vitamin E was not associated with a reduced prostate cancer risk overall but it was associated with a reduced risk for advanced prostate cancer (regionally invasive or distant metastatic, n = 123) (HR 0.43, 95 % CI: 0.19–1.0 for 10-year average intake ≥400 IU/day vs. non-use) [80]. In a prospective study of plasma vitamin E and prostate cancer mortality, there was a reduced risk associated with higher circulating levels limited to smokers, although the number of cases was small (<30) [81]. Other epidemiological studies have similarly found a protective effect limited to smokers [82–84].
The SELECT and PHS II trials were done in the PSA screening era, and had small numbers of current smokers. Thus neither trial could address the effect of alpha-tocopherol specifically on advanced or fatal prostate cancers, or among current smokers. However, the results overall do not support the use of supplemental vitamin E for prostate cancer prevention.
The trace element selenium is not an anti-oxidant per se, but plays an important role as an essential element for the antioxidant enzyme glutathione peroxidase [85] as well as other selenoproteins involved in exerting anti-tumor effects [86, 87]. Dietary intake of selenium depends on the selenium content of soil in which foods are grown, which varies greatly by geographic area. Ecologic studies have suggested an inverse association between selenium soil content and prostate cancer incidence [88]. Because selenium content in specific foods vary as a function of the selenium content of the soil, epidemiological studies of selenium require biomarkers, primarily measuring levels in blood or toenails. Since the activity of some selenoenzymes plateau with higher selenium level [89], the chemopreventive effect of selenium may be greatest in populations with low selenium exposure [90].
The Nutritional Prevention of Cancer Trial found a 63 % reduction in prostate cancer risk among men taking selenium supplements [91]; with additional follow-up time, the protective effect was limited to those with low baseline levels of PSA or selenium [90]. Another trial of selenium (with vitamin E and beta-carotene) found a reduction in total cancer mortality in China [76]. The SELECT trial found no association between selenium and prostate cancer risk (RR 1.09, 99 % CI: 0.93–1.27; P = 0.18). Moreover, baseline selenium levels were not associated with total prostate cancer risk, nor did levels modify the association between selenium supplementation and risk [92].
Six prospective biomarker studies have reported significant associations between higher levels of selenium and reduced prostate cancer risk [92–98], particularly for advanced disease [93, 94, 97], however, not all epidemiological studies have reported a protective association of selenium [99–101]. Furthermore, two randomized studies found no effect of selenium supplementation, alone or in combination, in reducing progression of high-grade prostatic intraepithelial neoplasia (PIN) to invasive cancer [102, 103].
In conclusion, there is some evidence that selenium may play a role in prostate cancer biology; however, there is no evidence to support the use of selenium supplements to prevent prostate cancer.
Lycopene and Tomato–Based Products The carotenoid lycopene is found in high quantities in tomato and tomato-based products, as well as pink grapefruit, and watermelon [104]. Lycopene accumulates in high levels in prostate tissue, and given its role as a potent antioxidant, is plausible as a potential protective factor for prostate cancer. This hypothesis has been tested in multiple studies that have investigated lycopene, or lycopene-rich food, such as tomato and tomato-based products, in relation to prostate cancer risk [105–125]. In a meta-analysis of studies published up to 2003 [126], high intakes of tomato or tomato-based products was associated with a 10–20 % reduction in prostate cancer risk. For the serum-or plasma-based studies, high concentrations of lycopene conferred a 25 % reduction in prostate cancer risk. More recent epidemiological studies of lycopene and prostate cancer showed mixed results, with some supporting an inverse association [118, 119, 127, 128], and others null [108, 109, 117, 120, 122, 129].
The association between tomatoes and prostate cancer has been studied extensively in the epidemiological literature, with evidence suggesting a significant benefit associated with a higher intake of tomatoes, particularly cooked tomatoes, or lycopene, the major antioxidant in tomatoes. In a meta-analysis of 10 prospective cohort or nested case-control studies, the relative risk of prostate cancer among consumers of higher amounts of raw tomato (5th quantile of intake) was 0.89 (95 % CI 0.80–1.00) [126]. For cooked tomato products, which are more bioavailable sources of lycopene than fresh tomatoes [130], the summary RR was 0.81 (95 % CI 0.71–0.92) comparing extreme categories of intake. The results from cohort studies generally indicate a 25–30 % reduction in risk of prostate cancer, whereas dietary-based case-control studies are not supportive of an association. For example, the summary RR for intake of one serving/day of raw tomato was 0.97 (95 % CI: 0.85–1.10) for the case-control studies and 0.78 (95 % CI: 0.66–0.92) for cohort studies [126].
The 2004 meta-analysis found an inverse association in studies of plasma lycopene and prostate cancer risk, with corresponding summary relative risks of 0.55 (95 % CI: 0.32–0.94) for case-control studies and 0.78 (95 % CI: 0.61–1.00) for cohort studies [126]. An additional nested case-control study not included in the meta-analysis found a modest, not statistically significant, inverse association overall, and a significantly reduced risk with higher levels among men over 65 years old and among those without a family history of prostate cancer [127]. However, more recent studies have found no associations with serum lycopene [118, 120, 122, 123, 129]. It is possible that these conflicting results are due, in part, to the changing mix of prostate cancer cases diagnosed with PSA screening [131], which has increased the pool of biologically indolent cancers.
Indeed, epidemiological studies generally point to a stronger reduction in risk of advanced stage or lethal prostate cancer, suggesting that tomato products and lycopene may play a role in prostate cancer progression. For example, in the HPFS, the associations comparing high and low quintiles of lycopene intake were 0.91 (0.84–1.00) for total prostate cancer and 0.72 (0.56–0.94) for fatal or metastatic disease [92]. This study also found that higher lycopene intake was associated with biomarkers indicating lower angiogenic potential in tumor specimens. In the EPIC study based on 966 total cases and 205 advanced stage cases of prostate cancer, there was no association between plasma lycopene and overall risk, but men in the top quintile of plasma lycopene had a significantly reduced risk of advanced stage prostate cancer (RR 0.40, 95 % CI: 0.19–0.88) [118].
Although not definitive, the available data suggest that increased consumption of tomato and tomato-based products is associated with lower prostate cancer risk and progression. Whether the effect is driven through lycopene or other aspects of tomatoes remains undetermined. The relationship appears to be stronger for advanced prostate cancer than for indolent disease.
9.3.3.5 Calcium, Dairy Products, and Vitamin D
Calcium intake has been associated with an increased risk of prostate cancer in many but not all epidemiological studies. A meta-analysis in 2005 found an increased risk of 1.39 (95 % CI = 1.09–1.77), for extreme categories of calcium intake [132]. Since the meta-analysis, four new prospective cohorts studies found some suggestion of an increased risk of prostate cancer with higher calcium [133–136] while five studies found no associations [109, 135, 137–140]. Total calcium intake varied widely across study populations: the highest category of intake was less than 1000 mg/day in three studies, whereas the highest category was greater than 2000 mg/day in three other studies [134, 135, 137–140]. Some, but not all, studies have reported stronger associations between high intake of calcium and risks of aggressive forms of prostate cancer, defined by high grade, or advanced or lethal prostate cancer [17, 141, 142].
The association between serum calcium and risk of prostate cancer also has been examined in several prospective studies. Serum calcium was associated with an increased risk of fatal disease in National Health and Nutrition Examination Survey (NHANES) I and NHANES III [143, 144] with a RR of fatal prostate cancer of 2.68 (95 % CI: 1.02–6.99). Similar increases in risk were seen for both total serum calcium and ionized serum calcium, the biologically active component. Two nested studies of Swedish men found no association between serum calcium and overall risk of prostate cancer [145, 146]; in fact, there was a weak inverse association with overall risk in one study [146]. This study also found no indication of an association between serum calcium and risk of fatal prostate cancer. Circulating calcium levels are tightly regulated and are related to diet only at very high levels of intake, so it is unclear how this finding relates to dietary calcium intake, if at all. However, it suggests a role for calcium, vitamin D, and perhaps related factors, such as parathyroid hormone, in the etiology of lethal prostate cancer.
Dairy foods, a major dietary source of calcium, have also been associated with risk, with a meta-analysis reporting a RR of 1.11 (95 % CI: 1.03–1.19) for total dairy, 1.06 (95 % CI: 0.91–1.23) for milk; and 1.11 (95 % CI: 0.99–1.25) per serving for cheese [132]. Most [133, 139, 147], but not all [135, 148] studies published since this meta-analysis have tended to support an association between higher milk or dairy consumption and total prostate cancer risk. However, findings specifically for advanced or lethal cancer are mixed [107]. The correlation between dairy foods and calcium and other nutrients creates challenges in trying to disentangle the independent effects of these compounds; however, studies that have tried to separate effects generally suggest calcium may be the predominant player in explaining positive associations with prostate cancer. As a result, the World Cancer Research Fund 2007 Expert Report on Diet and Cancer concluded that calcium is a “probable” risk factor for prostate cancer, while the evidence for dairy was weak/inconclusive [149]. Since then, the EPIC study found that dairy calcium, but not non-dairy calcium, was associated with total and high grade prostate cancer risk [133].
One proposed mechanism is that calcium acts by suppressing circulating levels of dihydroxyvitamin D (1,25(OH)2D), the bioactive metabolite of vitamin D. The main source of vitamin D is endogenous production in the skin resulting from sun exposure, and diet is a secondary source. 1,25(OH)2D is the most biologically active form, whereas 25(OH)D is found in much higher concentrations and better reflects sun and dietary exposure [150]. Dairy protein also increases levels of insulin-like growth factor (IGF) [151], which may thus influence risk of advanced or lethal prostate cancer [152].
No study of dietary or supplemental vitamin D have reported protective effects for prostate cancer incidence [153–156]. Results of studies using prediagnostic circulating vitamin D metabolites have reported mainly null results [157–169], in addition to significant positive [170] inverse [171], and U-shaped [172] associations. There is, however, a suggestion that vitamin D plays a role in prostate cancer progression. Genetic variants in the vitamin D pathway are associated with risk of recurrence or progression and prostate cancer-specific mortality [173]. In addition, high expression of the vitamin D receptor protein in prostate cancer tissue has been associated with lower risk of lethal cancer among men with prostate cancer in the HPFS and PHS [174]. Prostate cancer patients with the lowest levels of pre-diagnostic 25(OH)D had significantly greater risk of prostate cancer-specific mortality, with a RR of 1.59 (95 % CI: 1.06, 2.39) for the highest versus lowest quartiles [175]. Pre-diagnostic vitamin D levels were significantly associated with both stage and grade in this study. Thus, while vitamin D exposure does not seem to be associated with lower risk of incident prostate cancer, multiple lines of evidence suggest that the vitamin D pathway may play a role in prostate cancer progression.
9.3.3.6 Coffee
Most prior epidemiological studies of coffee and prostate cancer have focused on total incidence of disease, with generally null results. However, recent meta-analyses support an inverse association of coffee intake and risk of fatal or advanced disease [176–179]. Discacciati et al. [179] found a summary RR of 0.89 (95 % CI: 0.82–0.97) per 3 cups/day increment in coffee intake, as well as an inverse association with high grade (Gleason 8–10) disease. These intriguing data, while biologically plausible, need to be confirmed in additional study populations with large numbers of fatal or advanced disease.
Coffee is rich in several biologically active compounds including caffeine, minerals, and phytochemicals. In observational and animal studies, long-term coffee drinking has been associated with improved glucose metabolism and insulin secretion in observational and animal studies, and coffee is a potent antioxidant.
9.3.3.7 Statins
The class of lipid lowering medications known as statins have been proposed to have anti-tumor effects in prostate by influencing cell proliferation, inflammation, and steroidogenesis. The first study to look at the association was by Platz et al. [180] who found a RR of 0.39 (95 % CI: 0.19–0.77) of advanced prostate cancer comparing men who were statin users to nonusers. In a 2012 meta-analysis of 27 observational studies, the pooled RR of statins of 0.93 (95 % CI: 0.87–0.99) for total prostate cancer and 0.80 (95 % CI: 0.70–0.90) for advanced prostate cancer based on seven studies [181]. Since publication of the meta-analysis, six additional epidemiological studies have reported on associations between statin use and lethal prostate cancer, all suggesting inverse associations [182–185]. The largest to date included more than 11,000 prostate cancer patients in the United Kingdom and studied both prediagnostic and postdiagnostic statin use [186]. Post-diagnostic statins were associated with 34 % (95 % CI: 0.66–0.88) lower risk of prostate cancer death, and the effect was stronger among men who were using statins before diagnosis. Additional studies are needed to disentangle the relevant etiological window as well as identify mechanisms of association.
9.4 Summary
Prostate cancer epidemiology is complex, in part due to the biological heterogeneity of the disease as well as PSA screening. The established risk factors for prostate cancer incidence—age, race/ethnicity, family history, and genetic variants—are not modifiable, and thus primary prevention of prostate cancer is challenging. However, there are a number of promising lifestyle and dietary factors that may lower risk of developing a more aggressive cancer or hold promise in secondary prevention among prostate cancer patients.
References
2.
Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, et al. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014;65(2):467–79.PubMed
3.
Bill-Axelson A, Holmberg L, Garmo H, Rider JR, Taari K, Busch C, et al. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med. 2014;370(10):932–42.PubMedPubMedCentral
4.
Loeb S, Bjurlin MA, Nicholson J, Tammela TL, Penson DF, Carter HB, et al. Overdiagnosis and overtreatment of prostate cancer. Eur Urol. 2014;65(6):1046–55.PubMedPubMedCentral
5.
Lin CC, Gray PJ, Jemal A, Efstathiou JA. Androgen deprivation with or without radiation therapy for clinically node-positive prostate cancer. J Natl Cancer Inst. 2015;107(7).
6.
Sweeney CJ, Chen YH, Carducci M, Liu G, Jarrard DF, Eisenberger M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373(8):737–46.PubMedPubMedCentral
7.
Gillessen S, Omlin A, Attard G, de Bono JS, Efstathiou E, Fizazi K, et al. Management of patients with advanced prostate cancer: recommendations of the St. Gallen advanced prostate cancer consensus conference (APCCC) 2015. Ann Oncol. 2015;26(8):1589–604.PubMedPubMedCentral
8.
Ferlay J, Bray F, Pisani P, Parkin MX. GLOBOCAN 2002: cancer incidence, mortality and prevalence worldwide. Lyon: IARC Press. Report No. 2004.
9.
GLOBOCAN 2008 cancer fact sheet: prostate cancer incidence and mortality worldwide in 2008 summary [Internet]. 2008. Available from http://globocan.iarc.fr/factsheets/cancers/prostate.asp. Cited 16 Nov 2011.
10.
SEER cancer statistics review 1975–2008. Bethesda, MD: National Cancer Institute. http://seer.cancer.gov/csr/1975_2008/, based on November 2010 SEER data submission, posted to the SEER web site [Internet]. 2011.
11.
Shimizu H, Ross RK, Bernstein L, Yatani R, Henderson BE, Mack TM. Cancers of the prostate and breast among Japanese and white immigrants in Los Angeles County. Br J Cancer. 1991;63(6):963–6.PubMedPubMedCentral
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree