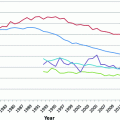
Fig. 13.1
Ovarian cancer incidence and mortality in the United States, 2000–2012 (data from surveillance, epidemiology, and end results program)
13.2.1.2 Geographic Variation
Ovarian cancer incidence is higher in developed than developing countries with the highest rates in Europe, North America, and Australia and lowest rates in Africa, after accounting for age (Fig. 13.2). Interestingly, while ovarian cancer is relatively rare in Asia, most cases from this region are clear cell tumors [44, 45], which often have a poor prognosis since they generally do not respond well to standard chemotherapy at advanced stages [46].
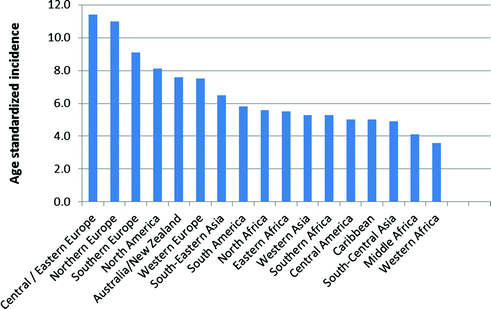

Fig. 13.2
Worldwide variation in ovarian cancer incidence (data from Globocan 2012, available at http://globocan.iarc.fr)
13.2.1.3 Variation by Age
Epithelial ovarian cancer is rare at younger ages, and increases steadily starting in a woman’s reproductive years (Fig. 13.3). Most ovarian cancer cases are diagnosed after menopause with a median age at diagnosis of 63 years [38].
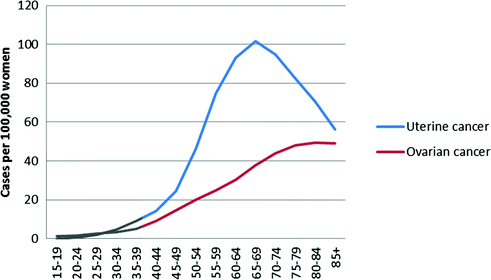

Fig. 13.3
Age-specific incidence rates for ovarian and endometrial cancer, United States, 2008–2012 (data from surveillance, epidemiology, and end results program)
13.2.2 Endometrial Cancer
13.2.2.1 Trends Over Time
Endometrial cancer incidence in the U.S. has remained relatively steady since 2000 (Fig. 13.4), with an age-adjusted estimate of 24.8 endometrial cancer cases per 100,000 women in 2000, and 27.5 in 2012. The slight increase in recent years may be attributable to increasing average body mass index (BMI), which is expected to continue rising in the future.
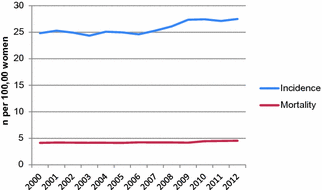

Fig. 13.4
Endometrial cancer incidence and mortality in the United States, 2000–2012. (data from surveillance, epidemiology, and end results program)
13.2.2.2 Geographic Variation
Endometrial cancer is the most common gynecologic cancer in developed areas like North American and Europe [47], and second in developing areas, like Africa, where endometrial cancer incidences follow behind cervical cancer (Fig. 13.5).
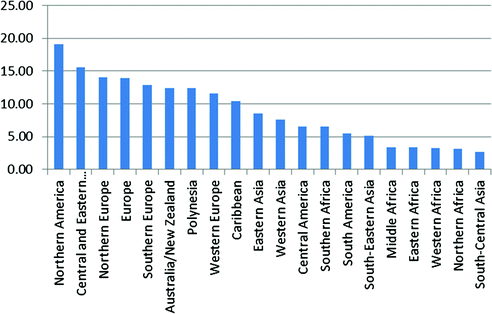

Fig. 13.5
Worldwide variation in endometrial cancer incidence (data from Globocan 2012, available at http://globocan.iarc.fr)
13.3 Risk Factors
13.3.1 Ovarian Cancer
13.3.1.1 Reproductive History
The strongest and most consistent “risk” factors for ovarian cancer are oral contraceptive (OC) use and parity—both of which reduce ovarian cancer risk. These two factors also reduce the lifetime number of ovulatory cycles, which inflict repeated damage and repair to the ovarian surface that may initiate or promote carcinogenesis (Fig. 13.6). In a meta-analysis of 55 epidemiological studies, women who had ever used OCs had more than a 25 % reduction in risk (Odds Ratio (OR) 0.73, 95 % CI 0.66–0.81). Longer duration was correlated with greater reduction in risk in a dose–response fashion with the greatest reduction in risk for women who had used OCs more than 10 years (OR 0.43, 95 % CI 0.37–0.51) [48]. Since OC use is common in reproductive aged women, the population impact of this significant reduction in risk is notable for this rare disease with an estimated two ovarian cancer diagnoses and one ovarian cancer death avoided before age 75 for every 5000 woman-years of OC use [49]. To date, the reduction in ovarian cancer risk with OC use has persisted across calendar time despite the changes in formulations [49] and generally across ovarian cancer subtypes though there may be a greater reduction in risk for more aggressive tumors [50, 51].
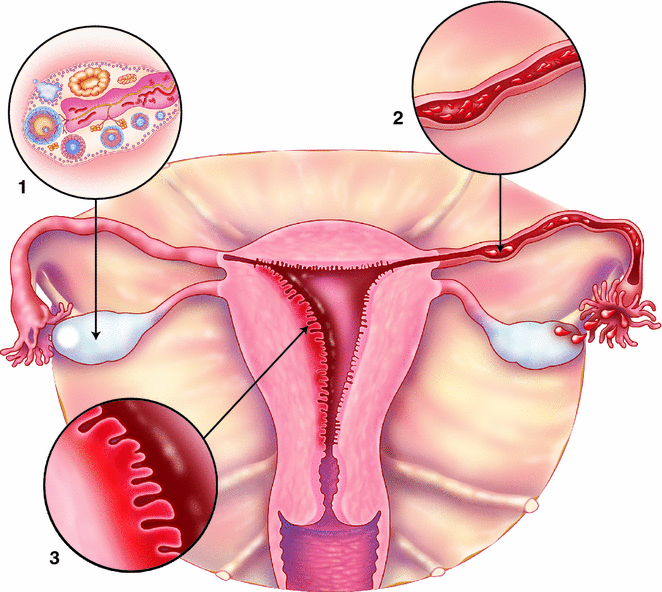

Fig. 13.6
Epidemiologic evidence that supports etiologic hypotheses for ovarian and endometrial carcinogenesis. 1 Increased ovarian cancer risk due to a greater number of ovulatory cycles is supported by decreased risk with pregnancy and oral contraceptive use, 2 Increased risk of endometrioid and clear cell ovarian cancer due to retrograde menstruation is supported by increased risk of those subtypes for women with endometriosis and decreased risk with tubal ligation, 3 Increased risk of endometrial cancer developing from estrogen induced endometrial hyperplasia is supported by an increased risk with estrogen-only hormone therapy and higher body mass index
While parous women have a lower ovarian cancer risk than nulliparous women, all pregnancies are not equally protective. A single pregnancy lowers ovarian cancer risk by approximately 40 % while each subsequent pregnancy lowers risk by an additional 10–15 % per pregnancy [52, 53]. Interestingly, parity is more protective for endometrioid, clear cell, low-grade serous, and mucinous tumors than high-grade serous, though studies differ on whether it is the first or subsequent pregnancies that differ [50, 54, 55]. Later age at first and last births reduce ovarian cancer risk [56–58]. However, later age at first birth is likely only protective due to its correlation with age at last birth since only age at last birth remains significantly associated with ovarian cancer risk when both are included in a multivariate model [58].
13.3.1.2 Hormone Replacement Therapy
The association between hormone replacement therapy (HRT) and ovarian cancer has been inconsistent and may vary by HRT formulation and ovarian cancer histology. Although prior pooled analyses and meta-analyses reported no association between HRT use and ovarian cancer risk [59–64], some studies suggest that long duration of HRT, particularly estrogen-only formulations, can increase risk [65–68]. For instance, in the Nurses’ Health Study, women who used HRT for more than 5 years had a significant 40–50 % increase in ovarian cancer risk compared to women who had never used HRT [62]. In the United Kingdom (U.K.) Million Women’s Study, increased risk with HRT use was restricted to women who were current users [63].
13.3.1.3 Endometriosis
Endometriosis, which affects approximately 10% of reproductive aged women, is the occurrence of endometrial-like tissue outside the uterus that is often associated with pain and infertility [69–71]. Women with endometriosis have three times the risk of developing clear cell ovarian cancer and twice the risk of developing endometrioid or low-grade serous ovarian cancer as women without endometriosis [72]. On pathologic review, ovarian tumors are sometimes found adjacent to endometriosis and these tissues share genetic mutations and mRNA expression patterns [73–75].
A pooled analysis from the Ovarian Cancer Association Consortium showed increased risk of clear cell (OR 3.1, 95 % CI 2.4–3.8), endometrioid (OR 2.0, 95 % CI 1.7–2.5) and low-grade serous (OR 2.1, 95 % CI 1.4–3.2) ovarian cancer for women with self-reported endometriosis, compared to the general population but not of high-grade serous or mucinous ovarian cancer [72].
13.3.1.4 Tubal Ligation
Tubal ligation, surgical sterilization by closure of the fallopian tube, reduces a woman’s overall ovarian cancer risk by 30–34 %, according to recent meta-analyses [76, 77]. Interestingly, this association has held up across time, despite changes in the how tubal ligations have been performed (cutting, burning, banding) [76, 78], though a recent study suggests that tubal sterilization by excision may be more protective than other methods [79]. Tubal ligation is thought to reduce ovarian cancer risk by blocking retrograde menstruation or inflammatory contaminants from ascending the reproductive tract (Fig. 13.6) and thereby preventing exposure of the ovaries to these potential carcinogens, such as talc and endometrial tissue [80]. Recently, many studies and pooled analyses have demonstrated that the reduction in risk afforded by tubal ligation is restricted to endometrioid and clear cell types [81, 82]. In a pooled analysis including 10,157 cases from the Ovarian Cancer Association Consortium, Sieh et al. observed that women with a tubal ligation had a 29 % reduction in serous ovarian cancer risk, but a 52 and 48 % reduction in risk for endometrioid and clear cell subtypes, respectively [82].
13.3.1.5 Genital Powder Use
Powder use, which generally includes talc, is associated with a 25–35 % increase in ovarian cancer risk when applied to the genital area [83, 84]. While the association between ever use of genital powder and ovarian cancer is fairly consistent over 20 years of research, evidence of a dose response has been inconsistent [85, 86], which has brought into doubt the biologic plausibility of the association.
13.3.1.6 Smoking
The association between smoking and ovarian cancer differs by histologic subtype. In an analysis within the Ovarian Cancer Association Consortium, including more than 14,000 cases, current smoking was associated with a 30 % increase in mucinous invasive, but not other histologic subtypes [87]. Former versus never smoking was associated with a 30 % increase in risk of serous borderline ovarian cancer. Interestingly, current smoking was associated with a reduced risk of endometrioid (OR 0.84, 95 % CI 0.69–1.02) and clear cell ovarian cancer (OR 0.74, 95 % CI 0.56–0.98), which is not entirely surprising since smoking also decreases the risk of endometriosis and endometrial cancer.
13.3.1.7 Body Mass Index
Observations regarding BMI and ovarian cancer risk are inconsistent. A pooled analysis of 12 cohort studies, including 2036 cases, showed no significant increase in ovarian cancer risk with each 4 kg/m2 increase in prediagnostic adult life BMI (OR 1.01, 95 % CI 0.95–1.07) [88]. In contrast, a pooled analysis of 11 case-control studies in OCAC, including 13,548 cases and 17,913 controls reported a small, but statistically significant increase in risk with each 5 kg/m2 increase in BMI for both invasive (OR 1.04, 95 % CI 1.00–1.08) and borderline tumors (OR 1.18, 95 % CI 1.14–1.23) [89]. Furthermore, the association with BMI was restricted to mucinous and endometrioid tumors. These results are consistent with a meta-analysis of 47 studies showing a statistically significant increase in ovarian cancer risk with each 5 kg/m2 increase in BMI in both prospective (Relative Risk (RR) 1.03) and population-based case-control studies (RR 1.10). However, the association between BMI and ovarian cancer risk per 5 kg/m2 differed by use of HRT use with a positive association between BMI and risk in never users (RR 1.10, 95 % 1.07–1.13) and an inverse association in ever HRT users (RR 0.95, 95 % CI 0.92–0.99) [90].
13.3.1.8 Polycystic Ovarian Syndrome
Recent meta-analyses disagree on whether ovarian cancer risk is higher in women with polycystic ovarian syndrome (PCOS), a condition characterized by irregular menstrual cycles, infertility, hyperandrogenism, and frequently a higher BMI. One epidemiological study reported a two- to threefold increased risk associated with PCOS [91], while another study found no significant association [92]. Differences might be explained by variability in the classification of PCOS or considerations of potential confounders like OCs, which are often a first line therapy to treat irregular menstrual cycles.
13.3.1.9 Family History
There are a number of inherited genetic risk factors emerging for ovarian cancer. Having a mother or sister with the disease increases a woman’s risk for ovarian cancer by approximately two- to threefold [93]. Most hereditary ovarian cancers are due to BRCA1 and BRCA2 mutations, accounting for 10–15 % of ovarian cancers [94–98]. Less commonly, hereditary ovarian cancer occurs due to the Lynch syndrome or hereditary non-polyposis colorectal cancer syndrome (HNPCC), which is characterized by mutations in mismatch repair genes (hMLH1, hMSH2, hPMS1, hPMS2, and hMSH6). These syndromes account for about 2 % of ovarian cancers [94]. While these higher penetrant genetic factors are more likely to be found in families in which a number of relatives have been affected with breast or ovarian cancer, an estimated 10 % of “sporadic” ovarian cancer [99] and up to 40 % among women with a Jewish ethnic background [100] also carry these genetic variants.
13.3.2 Endometrial Cancer
Unlike ovarian cancer, endometrial cancer risk factors can be largely explained by a single mechanism—excess estrogen exposure that promotes endometrial hyperplasia that can transform into endometrial cancer (Fig. 13.6). For instance, estrogen therapy unopposed by progestin is one of the strongest risk factors for endometrial cancer, which is why this type of hormone therapy is only recommended for women who have had a hysterectomy.
13.3.2.1 Reproductive History
Most reproductive factors that influence endometrial cancer risk can be attributed to increased estrogen exposure. For example, earlier age at menarche and later age at menopause both extend the duration of exposure to ovarian hormones and increase endometrial cancer risk [101]. Interestingly, a later age at last birth lowers endometrial cancer risk and largely explains the inverse association between number of children and endometrial cancer risk [101]. In an international pooled analysis including 8671 cases and 16,562 controls, Setiawan et al. reported an 18 % decrease in endometrial cancer risk for each 5-year increase in age at last birth [102].
13.3.2.2 Hormone Replacement Therapy
The strong and consistent elevation in endometrial cancer risk with HRT has lead to recommendations that women with an intact uterus avoid estrogen-only therapy. In a meta-analysis of 30 studies, estrogen-only users had a twofold increase in endometrial cancer risk and nearly a tenfold increase in risk for women who had used estrogen-only therapy for more than 10 years [103].
13.3.2.3 Body Mass Index
Given the growing obesity epidemic, the strong and consistent association between increasing BMI and endometrial cancer risk is concerning and may explain a large proportion of the recent rise in endometrial cancer incidence in the U.S. [104]. A meta-analysis of 24 prospective studies, including more than 17,000 cases, reported a 60 % increase in risk for each 5 kg/m2 increase in BMI. Compared to normal weight women (BMI 20–25 kg/m2), the heaviest women (BMI > 42 kg/m2) had more than a ninefold increase in risk. The association was strongest among women with a BMI > 42 kg/m2 who had never used HRT (RR 20, 95 % CI 8–52). Estrogenic proliferation is thought to be the primary mechanism underlying the BMI endometrial cancer association since fat cells convert androgens to estrogens and are the leading source of estrogen in postmenopausal women. A stronger association in non-HRT users lends support to this mechanism.
However, associations between diabetes and/or metabolic syndrome, which consists of a combination of cardiovascular risk factors including dysglycemia, elevated blood pressure, low high-density lipoprotein cholesterol levels, with endometrial cancer suggest that BMI may increase endometrial cancer mechanisms through non-estrogenic pathways as well. Recent meta-analyses reported a nearly twofold increase in endometrial cancer risk for women with diabetes or metabolic syndrome compared to those without [105]. While higher BMI largely accounts for the association between these diabetes and endometrial cancer, a significant association remains after adjusting for BMI [106], which suggests that insulin dysregulation or some other aspect of diabetes encourages endometrial carcinogenesis above and beyond increased body weight alone.
13.3.2.4 Smoking
Compared to women who have never smoked, former and current smokers have a 20–60 % reduction in endometrial cancer risk [107, 108]. Risk reduction is greater for current smokers (RR 0.65, 95 % CI 0.55, 0.78) than for former smokers (RR 0.89, 95 % CI 0.80, 1.00), but there is no dose response association with greater number of cigarettes per day [109]. Potential mechanisms for this reduction in risk relates to reduced or modified estrogen, including a younger age of menopause due to destruction of oocytes, lower BMI, a shift in estrogen metabolism towards the anti-carcinogenic 2-hydroxyestrone form, and increased progesterone [107, 109].
13.3.2.5 Polycystic Ovarian Syndrome
An elevated risk of endometrial cancer for women with PCOS, a condition in which women are often overweight and have insulin dysregulation, is not surprising given these are both established endometrial cancer risk factors. Women with PCOS have approximately a fourfold increase in risk of endometrial cancer [92, 110]. Although the association between PCOS and endometrial cancer is attenuated after adjustment for BMI, suggesting that some of the association is explained by higher BMI in women with PCOS, a significant association remains indicating that PCOS increases risk independent of increased body weight [110].
13.3.2.6 Family History
Having a mother or sister with endometrial cancer doubles a woman’s own risk [111], and there are several familial syndromes known to include endometrial cancer. Most notably, Lynch syndrome is associated with a 27–71 % lifetime risk of endometrial cancer compared to a 3 % risk in the general population [47]. Cowden syndrome is a rare familial syndrome, characterized by a mutation in Phosphatase and tensin homolog (PTEN), and is associated with 13–28 % lifetime risk of endometrial cancer [47, 112].
13.4 Conclusion
The last several years have been a time of great epidemiologic and basic science discovery for ovarian and endometrial cancer. These cancers of the reproductive tract have both concordant and discordant risk factor profiles. For the former, identification of distinct subtypes has resulted in paradigm-shifting focus for etiologic hypotheses as well as diagnostic and treatment discovery. For the latter, etiologic hypotheses which expand beyond estrogenic proliferation as risk factors that do not fit the classic etiologic framework, like age at last birth and PCOS, have been appreciated. As distinct subtypes of these cancers are identified, the need for multicenter international collaborations that contribute “big data” to foster disease subtype discovery will be critical to moving the field forward.
References
1.
American Cancer Society. Cancer facts and figures. Atlanta, GA: American Cancer Society; 2015.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree