In the U.S., lung cancer ranks second in estimated new cancer cases in men and women, after prostate and breast cancer, respectively. For 2015, an estimated 115,610 men will be diagnosed, which accounts for 14 % of all cancer new cases, and 105,590 women, accounting for 13 % of all cancer new cases. Lung cancer remains the first cause of cancer-related mortality for both sexes, accounting for 28 % of all cancer deaths in males and 26 % in women [60].
Similar patterns of incidence and mortality are seen in Europe [61]. Lung cancer is the leading cause of cancer-related death for males (25 % of all cancer deaths) and is predicted to be the leading cause of cancer-related death for women as well in 2015 (14 % of all cancer deaths) [62].
In China, lung cancer is the most common cancer in men and the second most common cancer in women after breast cancer, with 416,333 new male cases and 189,613 new female cases in 2010. It is the first cause of cancer-related death in both sexes [63].
Africa has the lowest incidence of lung cancer worldwide [59], but the increased prevalence of smoking in adolescents in Africa is of concern [64].
19.2.3 Trends Over Time
The incidence of lung cancer has varied considerably in populations over time, and is determined by smoking habits over time. Lung cancer was a rare disease up until the end of the nineteenth century, with few case reports published in the literature until 1900. The incidence started to increase significantly in the U.S. and other countries in the second half of the nineteenth century and the first decade of the twentieth century. In the 1930s, the first case-control studies indicated a correlation with smoking, and in the next decades several reports in Europe and U.S. were published regarding the lethal hazards of tobacco. Scientists from the Nazi Germany had recognized the addictive nature of tobacco and the associated potential hazard of lung cancer and an antismoking campaign was launched [65]. In 1950, some of the milestone epidemiological studies were published, particularly from Sir Richard Doll, the British epidemiologist, who interviewed patients with lung cancer and suggested that lung cancer risk is associated with the amount of cigarettes smoked and the duration of smoking [66], and from Wynder and Graham [67] in the U.S. who reported similar observations. In 1954, Sir Doll confirmed the association between smoking habits and lung cancer risk within the British Doctor’s Study, a prospective cohort of 40,000 doctors [68]. In 1962, the Royal College of Physicians issued a report on the association of smoking and lung cancer [69], and in 1964 the famous U.S. General Surgeon’s report was published and smoking was formally accepted as a definite cause for lung cancer [70]. A plethora of epidemiologic studies, animal experiments and pathologic studies have provided robust evidence that tobacco causes precancerous lesions in lung tissue [71].
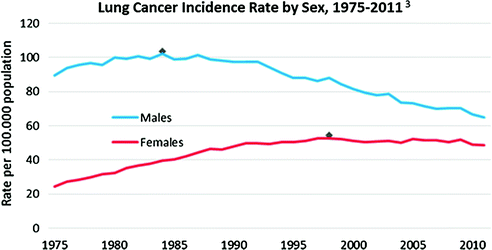
Lung cancer incidence in the U.S. continued to increase for males until the mid-1980s and then gradually started to decline. For women, due to later adoption of smoking reaching a peak two decades later, incidence continued to increase until late 1990s, and then followed a similar declining pattern [1] (Fig. 19.2).
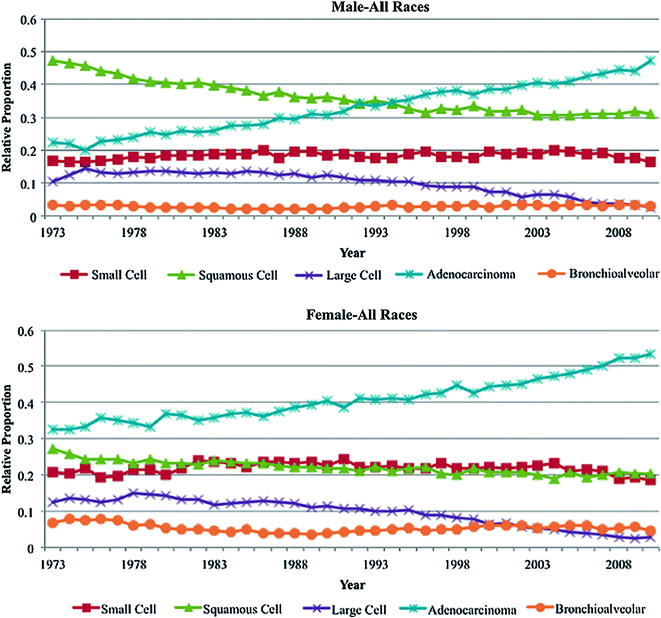
The frequency of specific subtypes of lung cancer has also changed during the years reflecting changes in smoking habits. Squamous carcinoma was the most common histologic type until the 1980s and then the incidence of squamous, small cell and large cell histology started to decline, whilst adenocarcinoma started to increase and now accounts for more than half of all cases [72] (Fig. 19.3). This increase was attributed to changes in smoking behaviour associated with the introduction of filtered cigarettes. These cause smokers to take longer and deeper inhalations in order to compensate for reduced inhaled nicotine concentrations, resulting in increased deposition of smoke in the peripheral lung where adenocarcinoma usually arises [73].
19.2.4 Differences by Sex
Globally, deaths from lung cancer continue to be more common in men than women since smoking continues to be more common in men. The absolute difference has become smaller over the years since lung cancer incidence peaked earlier for men whilst continued to increase for women [74].
There are no significant differences between men and women in the effect of smoking for a given life time exposure [75]. One significant difference by sex is the incidence of lung cancer amongst never smokers [76]. There are significant geographical variations in the proportion of lung cancer cancers among women that have never smoked, varying from 83 % in South Asia to 15 % in the U.S. Among women who are never smokers, passive smoking exposure at home in adult life and especially prolonged exposure of ≥30 years increases the risk of lung cancer compared to women without home exposure (HR 1.61, CI 95 % 1.00–2.58) [77]. Another risk factor is indoor pollution produced by burning wood and coal for cooking and heating. A retrospective study in China showed that the use of smoky coal compared to smokeless coal increases the risk for lung cancer by more than 30 fold [78].
There is a strong, positive association between hormone replacement therapy (HRT) with oestrogen and progestin and lung cancer risk, as shown from the analysis of several randomized trials. HRT compared to placebo increases the risk of lung cancer and this increase is proportional to the duration of exposure to HRT, with a 50 % increased risk for ≥10 versus <10 years of use (HR 1.48, 95 % CI 1.03–2.12), as well as an association with advanced stage at diagnosis [79].
Although in recent years adenocarcinoma has become the most common histology for both sexes, proportionally more women will be diagnosed with adenocarcinoma compared to men, with the opposite for squamous carcinoma [80]. Women are also more likely to have an EGFR mutation compared to men [17].
In several studies, women have better prognosis than men irrespective of stage at diagnosis and treatment. Data from SEER of more than 18,000 elderly patients with stage I and stage II NSCLC diagnosed during 1991–1999 showed that irrespective of treatment and after adjusting for confounding factors, women had significantly superior outcomes, including lung cancer-specific survival [81]. A meta-analysis of 39 studies and a total of 86,800 patients found that women had better overall survival regardless of stage, histology and smoking status [82]. Women worldwide have a longer life expectancy than men but this alone cannot explain the differences, and it seems that several other biologic factors contribute to this result, warranting further research in this field [83].
19.2.5 Racial Differences
An early observation was that lung cancer incidence and mortality was higher in African-American men compared to white men [84]. The U.S. Multiethnic Cohort examined racial/ethnic differences in lung cancer risk in five different ethnic groups in the U.S.: African-Americans, Japanese-Americans, native Hawaiians, Latinos and white men and women. They found no differences in risk for those who smoked more than 30 cigarettes per day, but for those who smoked less than 30 cigarettes per day there was an increased risk of lung cancer for African-Americans and native Hawaiians even after adjusting for occupational risk factors, diet and socioeconomic status [85]. Possible explanations include different smoking styles with deeper and longer inhalations for African-Americans, but also biological differences of the effect of carcinogens.
Higher mortality from lung cancer in African-Americans has been directly associated with socioeconomic status and lack of access to health services, lack of information and differences in cultural beliefs towards lung cancer, as well as access to care [86]. EGFR mutations vary significantly among races and have been reported as occurring in 10–16 % of Whites, 50–60 % of East Asians [18, 87] and 22 % of Indians [88].
19.2.6 Trends in Mortality
Over time there has been a steady, but small improvement in lung cancer survival. In the U.S., mortality has shown a steady decline over time, with a 2.6 % annual reduction in mortality for men and a 1.3 % reduction for women between 2002 and 2011 [89]. In the United Kingdom (U.K.), there was an increase in the 5-year-survival rate for the period 1971–2011, from 5 to 8 % in men and from 4 to 12 % in women [90].
19.3 Risk Factors
19.3.1 Introduction
Lung cancer is one of the most characteristic examples of a relationship between exposure and disease in all of epidemiological science. The major risk factor of smoking is now well-established, and a latency period measured in decades explains the relationship of incidence and mortality with smoking habits around the world and over time.
19.3.2 Smoking
More than 1 billion people smoke worldwide and 80 % of them are in low- and middle-income countries. Smoking is responsible for 6 million deaths per year worldwide. In 2008, the World Health Organization introduced the 6 MPOWER measures to fight the tobacco epidemic:
Monitor tobacco use and prevention policies
Protect people from tobacco use
Offer help to quit tobacco use
Warn about the dangers of tobacco
Enforce bans on tobacco advertising, promotion and sponsorship
Raise taxes on tobacco [91].
19.3.2.1 Cigarettes
Tobacco contains at least 98 known hazardous compounds that have been extensively studied [92], belonging to one of the following categories: polycyclic aromatic hydrocarbons, azaarenes, N-nitrosamines, aromatic amines, heterocyclic aromatic amines, aldehydes, miscellaneous organic compounds and inorganic compounds [93, 94].
Smokers have at least a 20-fold increased risk of developing lung cancer compared to lifelong non-smokers [95]. Lung cancer risk increases with the number of cigarettes smoked per day in an almost linear way [96], but duration of smoking has the strongest impact [97]. Small cell and squamous histology have the strongest association with smoking with essentially all cases associated with smoking whilst adenocarcinoma is the most common histology in non-smokers [98].
19.3.2.2 Cigars and Pipes
Cigars contain tobacco that is wrapped in a tobacco leaf. Their association with lung cancer has been well documented and they contain many of the carcinogens that are found in cigarettes. Specific carcinogens like nicotine, N-nitrosamines, benzene, benzopyrene, carbon monoxide and nitrogen oxide are found in higher levels in cigars than cigarettes due to the curing and fermentation process of cigar manufacture [99]. Cigars are associated with a lower overall risk for lung cancer compared to cigarettes, but risk varies according to intensity, duration and depth of inhalation. Moreover, lung cancer mortality risks for non-smokers range from 1.59 to 7.64, as compared to non-smokers [100]. Data from the U.S. report that cigar use has doubled between years 2000 and 2011, possibly explained by their portrayal in the media as a symbol of success and luxury [101].
Pipe smoking is most prevalent among the elderly and is associated with at least a fivefold increased risk of lung cancer, independent of cigarette smoking. Again intensity, duration and depth of inhalation are important factors that determine risk [102].
19.3.2.3 Marijuana
Marijuana contains carcinogens such as benzopyrene and benzanthracene in higher concentrations than cigarettes. It is usually smoked without a filter and with deeper and longer inhalations resulting in increased retention of tar in the lungs compared to cigarette smoking [103]. Endobronchial biopsies of individuals who smoke marijuana have shown histopathologic changes such as squamous metaplasia and cellular atypia which are known precursor lesions of lung cancer. However, epidemiological data linking marijuana smoking and lung cancer have been conflicting [104]. Several limitations have been recognized in most of these studies, including quality of data available for use of an illegal product, confounding factors such as cigarette smoking, confounding by age since marijuana is most prevalent in the young and short follow up. Notably, a Swedish cohort study of more than 49,000 men with a follow up of 40 years showed that lung cancer risk is doubled (HR 2.12, 95 % CI 1.08–4.14) for heavy marijuana users compared to non-users, even after adjusting for confounding factors such as tobacco and alcohol use, respiratory conditions and socioeconomic status. Heavy marijuana use was defined as more than 50 times during lifetime [105]. Further studies are warranted to answer questions regarding the risks of marijuana smoking, and there is a need for systematic collection of data regarding use. Public health initiatives to inform patients about potential hazards of marijuana smoking, including lung cancer, are required [106].
19.3.2.4 Electronic Nicotine Delivery Systems
Electronic cigarettes were launched in China in 2003 and then patented internationally in 2007. They consist of a battery-operated heating device and a nicotine cartridge; heat converts nicotine to a vapour which is inhaled by the user. Although electronic cigarettes appear less harmful since they do not contain the variety of carcinogens that are found in traditional cigarettes, long-term data regarding their safety are not available and there are many concerns since they are produced by different companies without any independent quality check [107]. Controversy exists regarding their use as smoking cessation aid, with some proponents advocating their use as being less harmful than tobacco products, with a counter-argument that they may encourage continuation in smokers who would otherwise be willing to quit. Electronic cigarettes are not approved by the U.S. Food and Drug Administration (FDA) as a smoking cessation aid, and many prefer to encourage patients to use evidence-based smoking cessation methods and inform smokers about the lack of data in this field [108]. The American Association of Cancer Research (AACR) and the American Society of Clinical Oncology (ASCO) published a policy statement in March 2015 to address these concerns [109].
19.3.2.5 Second-Hand Smoke
Second-hand smoke exposure was recognized much later than primary use as a lung cancer risk factor. The first two studies of passive smoking were published in 1981 and reported increased risk of lung cancer in non-smoking wives of heavy smokers [110, 111]. A meta-analysis of 55 studies calculated a relative risk for lung cancer of 1.27 for non-smoking women exposed to second-hand smoke from their spouses, compared to women not exposed to spousal environmental tobacco smoke [112]. It is estimated that 21,400 lung cancer deaths worldwide are attributable to passive exposure to tobacco products, which includes exposure at home, work and in public places [113].
19.3.2.6 Smoking Cessation
Smoking cessation reduces risk of lung cancer at any age of quitting resulting in added years of life expectancy, and advice on this subject can been viewed as one of the most cost-effective of all healthcare interventions made by physicians [114–116]. The risk of lung cancer decreases with years of abstinence, although it never becomes identical to that for non-smokers even 40 years after smoking cessation [117]. Smoking reduction is also beneficial and results in reduction of lung cancer risk [118]. Smoking cessation is beneficial even after lung cancer diagnosis. Continuing smoking following treatment for early lung cancer is associated with an increased risk of recurrence, second primary tumours and all-cause mortality [51].
19.3.3 Radiation
It is well established that lung cancer risk is significantly increased in patients treated for Hodgkin’s lymphoma, with a median relative risk that ranges from 2.2 to 7 [119]. Both radiotherapy and chemotherapy with alkylating agents associated with treatment of Hodgkin’s increase the risk. The risk is related to dose of therapies, whilst combination of chemotherapy and radiotherapy acts synergistically in this context. In a large population-cohort study that followed more than 19,000 Hodgkin’s lymphoma survivors, median time to diagnosis of lung cancer after treatment for lymphoma was 10 years (range 1–28 years). The risk is even greater for smokers, with the highest risk in those who are moderate to heavy smokers, and were treated with both radiotherapy and chemotherapy (relative risk 49.0) [120].
For women treated for breast cancer, adjuvant radiotherapy post-mastectomy almost doubles the risk of subsequent ipsilateral lung cancer. Smoking is again the most important concomitant risk factor, and women who are ever smokers have almost a 40-fold increased risk compared to non-smokers who received adjuvant radiotherapy [121]. Lung cancer typically develops at least ten years after radiotherapy treatment for breast cancer [122].
19.3.4 Occupational Exposure
According to the International Agency for Research in Cancer (IARC), lung cancer is the most common occupational cancer worldwide [123]. Several occupations are associated with an increased lung cancer risk, including work involved in aluminium production, coal gasification, coke production, hematite mining, iron and steel founding, painting and rubber production [124]. It was estimated that 102,000 lung cancer deaths in the year 2000 could be attributed to occupational carcinogens [125], and every tenth lung cancer death is related to work-related factors [126].
Asbestos, radon and silica are the most common lung cancer carcinogens, but there are several others chemicals and mixtures like bis-(chloromethyl) ether and chloromethyl methyl ether, coal tar pitch, soot, sulphur mustard, diesel exhausts and metals like arsenic, beryllium, cadmium, chromium, nickel and their compounds [124].
Asbestos is characterized by flame resistance and has been used widely in the building and manufacturing industries [127]. Exposure increases the risk of lung cancer by at least threefold, and smoking acts synergistically [128, 129]. The risk is proportional to the concentration and frequency of the exposure [130].
Radon is a radioactive gas produced during the decay of uranium and is associated with a threefold increased risk of lung cancer in uranium miners [128]. Air pollution from radon of geological origin, which can accumulate indoors, has also been linked with risk of lung cancer in certain geographical areas [131].
19.3.5 Environmental Exposure
Air pollution in urban areas is associated with an increased risk of lung cancer [132]. The European Study for Cohorts for Air Pollution Effect was a large prospective study which confirmed that exposure to particulate matter air pollution contributes to lung cancer risk [133]. Indoor pollution from burning coal or cooking oil fumes without appropriate ventilation, which is common in less-developed countries, has also been linked with an increased risk especially in non-smoking women [76].
Water contaminated with high concentrations of arsenic has been linked with various types of cancer including lung cancer [134]. A characteristic epidemiologic example has been described in the Antofagasta area of Chile, where water was supplied by rivers arising from springs in the Andes with a high concentration in arsenic. A change in the water supply in 1958 led to a large increase in arsenic concentration in drinking water. For the period 1958–1970, a rise in the number of deaths from lung and bladder cancer was observed and linked to the high levels of arsenic in drinking water. In 1971, water treatment plants were installed and arsenic levels were lowered. Despite this change, cancer deaths continued to increase until the late 1990s, but then started to decrease, indicating a long latency period [135]. Cancer deaths attributed to arsenic exposure have also been described in Taiwan, Bangladesh and West India, and the World Health Organization has published guidelines regarding drinking water quality requirements [136].
19.3.6 HIV
Individuals with HIV infection have a threefold increase of risk for lung cancer compared to the general population. In contrast with AIDS-defining malignancies, for which incidence has decreased since the introduction of highly active antiretroviral therapy (HAART), lung cancer risk has increased among HIV-infected individuals, and this is attributed to the increase in life expectancy and aging of this population. Smoking remains the most significant risk factor for individuals with HIV infection, who have a higher smoking prevalence than the general population [137]. Despite these high smoking rates, additional factors seem to contribute to lung carcinogenesis, possibly including chronic pulmonary inflammation associated with repeated infections [138].
Introduction of HAART has resulted in survival improvements for AIDS-defining malignancies, but this is not the case for lung cancer where outcomes remain poor and unchanged over time [139]. Various studies have reported worse prognosis in HIV-infected lung cancer patients compared to non-HIV infected (HR 1.28, 95 % CI 1.17–1.39) even after adjusting for stage and treatment, indicating that immunosuppression may contribute to the more aggressive behaviour of the disease [140, 141].
19.3.7 Benign Lung Disease
Inflammation has been recognized as one of the hallmarks of cancer [142] and previous lung disease predisposes to lung cancer. A pooled analysis of 17 studies from the International Lung Cancer Consortium looked at the relationship of lung cancer with emphysema, chronic bronchitis, pneumonia and tuberculosis. Relative risk of lung cancer after adjusting for smoking was 2.44 for emphysema, 1.47 for chronic bronchitis, 1.57 for pneumonia and 1.48 for tuberculosis, as compared to those without any of these benign lung diseases [143].
Chronic obstructive pulmonary disease (COPD) and its subtypes chronic bronchitis, emphysema and chronic obstructive asthma, is a common disease in smokers, although 20 % of patients with COPD do not have a history of smoking [144]. Among never smokers, both emphysema and the combination of emphysema and chronic bronchitis together are associated with increased lung cancer risk, but not chronic bronchitis alone [145].
Pulmonary fibrosis is associated with a poor prognosis (median survival 2–3 years), with the most common cause of death being respiratory failure arising from the disease itself [146]. Several studies have shown that patients with pulmonary fibrosis have a higher risk of developing lung cancer with an incidence that ranges from 4.8 to 48 % [147–150]. A retrospective study showed a cumulative incidence of lung cancer at 1, 5 and 10 years of 3.3, 15.4 and 54.7 %, respectively [151].
Alpha1-antitrypsin deficiency carriers (heterozygous) may not have severe symptoms and may often be undiagnosed; however, they appear also to have an increased risk of lung cancer compared to non-carriers, with an odds ratio of 1.7 (95 % CI 1.2–2.4) [152].
19.3.8 Genetic Factors
Individuals with a first-degree relative with lung cancer have a 1.5-fold increase of lung cancer after adjustment for confounding factors, with a higher risk when a sibling rather than a parent is affected, as shown in a pooled analysis including 24 case-control studies from the International Lung Cancer Consortium. As expected, family history is a stronger risk factors for smokers compared to non-smokers, with more than a threefold difference [153]. Diagnosis of lung cancer in more than one relative, or in a relative at a younger age, have also been associated with increased risk, although different age cut-offs have been used in the various studies [154].
19.3.9 Diet
In the early 1980s, two large randomized studies with beta-carotene supplementation were conducted to examine its potential as chemoprevention. The CARET study (Beta-Carotene and Retinol Efficacy Trial) recruited more than 18,000 American men and women who were current or recent ex-smokers, or men with asbestos exposure [157]. The ATBC [158] (Alpha-Tocopherol Beta-Carotene Cancer Prevention) trial in Finland studied more than 23,000 male current smokers. Both of these trials actually showed a detrimental effect, with increased lung cancer incidence in individuals randomized to beta-carotene compared to placebo (by 28 % in the CARET trial and 16 % in ATBC). Interestingly, in the Physicians’ Health Study, which included more than 22,000 male physicians of whom half were never smokers, neither harm or benefit was found with beta-carotene supplementation [159]. There are several cohort, case-control and ecological studies that are looking into the protective effect of fruit consumption against lung cancer. Most of the studies report a protective effect for higher versus lower fruit intake; however, a significant limitation for all of these studies is that very few have adjusted for smoking status, highly likely to be a confounding variable in this context [160].
A meta-analysis of 20 prospective cohort studies showed that the risk of lung cancer is reduced by 3 % for every one serving per day of vegetables, 5 % for every one serving of fruit and 3 % for every one serving of fruit or vegetables. Risk was not reduced further after the threshold of two servings per day of fruit or vegetables [161]. Consumption of red meat has been linked with lung cancer and seems to have an adverse effect as shown in a meta-analysis of 34 studies, where it was estimated that there is almost a 35 % increase in lung cancer risk for the higher versus the lower consumption of red meat. Interestingly, all of the studies included were adjusted for smoking or involved non-smokers [162].
There is some evidence supporting physical activity as having a protective effect against lung cancer, including case-control and cohort studies, although there is significant heterogeneity in this data. Physical activity is a general term that is challenging to measure. Physical exercise may arise from occupational, household, transportation or recreational activities, and variability also arises from factors like frequency, intensity and duration [163]. In a prospective study of more than 500,000 men and women, Leitzmann et al. found a 23 % reduction in lung cancer risk for current smokers, and a 22 % reduction for ex-smokers, when comparing individuals with the highest and lowest levels of physical activity (≥5 times per week versus inactive). No difference was seen in never smokers. Physical activity was defined as any activity with duration ≥20 min that resulted in increased heart rate, respiratory rate or sweating [164]. A meta-analysis of 12 cohort studies and 6 case-control studies, including a total of almost 2.5 million participants, showed that any amount of physical activity compared to no activity resulted in a relative risk of 0.79 for lung cancer (95 % CI 0.68–0.86). Similarly to previously reported studies the benefit was seen in smokers and former smokers but not in never smokers [165].
19.3.10 Summary
Lung cancer accounts for millions of deaths every year worldwide. Despite recent advances in understanding the molecular mechanisms behind its pathogenesis, outcomes remain poor. Smoking is responsible for the majority of lung cancer cases and smoking cessation is the most cost-effective prevention measure.
References
1.
Howlader NNA, Krapcho M et al. SEER Cancer Statistics Review, 1975–2012, National Cancer Institute. Bethesda, MD. http://seer.cancer.gov/csr/1975_2012/, based on November 2014 SEER data submission, posted to the SEER web site, April 2015.
3.
Midthun DE. Overview of the risk factors, pathology and clinical manifestations of lung cancer. In: UpToDate, Post TW, editors. Waltham, MA: UpToDate. Assessed on 20 July 2015.
4.
Vansteenkiste J, Crino L, Dooms C, Douillard JY, Faivre-Finn C, Lim E, et al. 2nd ESMO consensus conference on lung cancer: early-stage non-small-cell lung cancer consensus on diagnosis, treatment and follow-up. Ann oncol (Official Journal of the European Society for Medical Oncology/ESMO). 2014;25(8):1462–74.
5.
Silvestri GA, Gonzalez AV, Jantz MA, Margolis ML, Gould MK, Tanoue LT, et al. Methods for staging non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of chest physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e211S–50S.PubMed
6.
Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of chest physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e142S–65S.PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree