Breast cancer is the most common cancer among women both in the United States and in the world. It is the second leading cause of cancer death among women in the United States. Among U.S. women in 2009, approximately 192,370 new cases of invasive breast cancer and 62,280 carcinoma in situ will occur and 40,170 will die from breast cancer.1 In terms of its global burden, there are about 1.05 million new cases and 373,000 deaths each year worldwide.2
There is a 4- to 5-fold variation in breast cancer incidence rates worldwide, with the highest in North America (99.4/100,000) and the lowest in Asia (22.1/100,000) and Africa (23.4/100,000).3 However, mortality rates are relatively less variable, with Africa (16.2/100,000) being similar to North America (19.2/100,000). These international variations are partly due to differences in environmental and lifestyle factors, screening practices, and treatment strategies.
In the past 50 years, breast cancer incidence has increased worldwide, including in the United States, where the highest rate is found.4 Data from the Surveillance, Epidemiology, and End Results (SEER) program show that incidence increased in the 1980s and 1990s in the United States but decreased after 2002 mainly in white women4 (Fig. 4-1). During the same period, 5-year survival also increased, reaching 90% at year 1997 for women 50 years or older and 87% for women under 50 at diagnosis (Fig. 4-2).4 The continued improvement in prognosis may be attributable to both screening, which increases the diagnosis of small, localized breast tumors, and treatment effectiveness.
Figure 4-1

Trends in female breast cancer incidence and death rates by race and ethnicity, United States. Rates are age-adjusted to the 2000 U.S. standard population. (Data from Ries L, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2005. Bethesda: National Cancer Institute; 2008.)
Figure 4-2
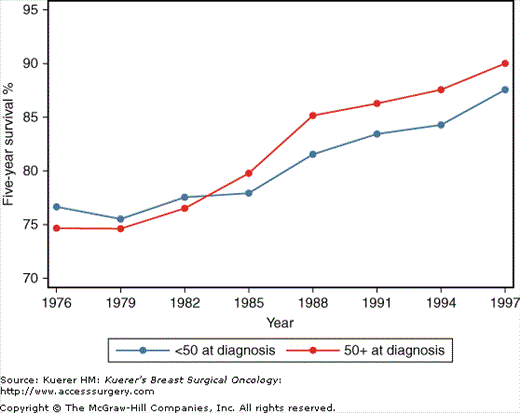
Breast cancer survival trends in the United States. Values shown are 5-year relative survival (survival adjusted for life expectancy—an approximation to breast cancer-specific survival). (Data from Ries L, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2005. Bethesda: National Cancer Institute; 2008.)
Breast cancer incidence rates increase with age, as shown by the log scale plot of age-specific cancer incidence rates in 5-year age groups (Fig. 4-3). For estrogen receptor (ER)-positive cancer, the incidence rates increase rapidly until approximately age 50 (proportional to the seventh power of the age), and then rise slowly (proportional to the first power of the age). However, the incidence rate of ER-negative cancer increases rapidly before age 50 (proportional to the fifth power of the age) and then remains constant. As a result, ER-positive tumors are more likely to occur in postmenopausal women.
Figure 4-3
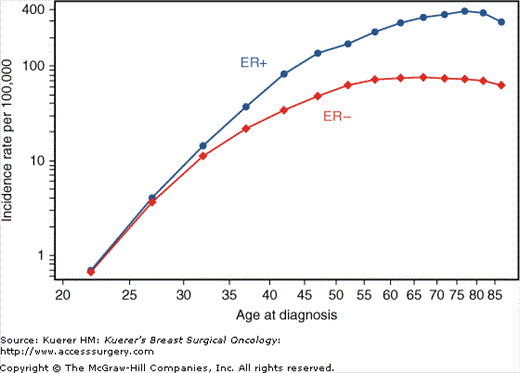
Breast cancer incidence rate by age and estrogen receptor status, United States, 2000-2005.4(Data from Ries L, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2005. Bethesda: National Cancer Institute; 2008.)
There are racial and ethnic differences in breast cancer incidence and mortality in the United States (Fig. 4-1). In the past 30 years, incidence rates were higher in white women than in African American women. However, African American women had higher mortality rates than white women, and this racial disparity has been widening in recent years. The incidence and mortality rates in Asian, Hispanics, and Native American women are lower than those of non-Hispanic white and African American women.
African Americans are more likely to have regional and distant disease, contributing to the survival disparity (Fig. 4-4A). However, African Americans also have lower 5-year survival rates than whites within stage strata (Fig. 4-4B). In addition to later stage at diagnosis, socioeconomic factors such as limited access to quality health care and comorbidities contribute substantially to outcome disparities.5,6 However, some degree of difference in outcomes persists between blacks and whites after accounting for these factors, suggesting that cancer biological differences might also be relevant.7,8 Breast cancers in African Americans are more likely to be early-onset, higher-grade, and ER-negative compared with those in whites.5,6
Figure 4-4
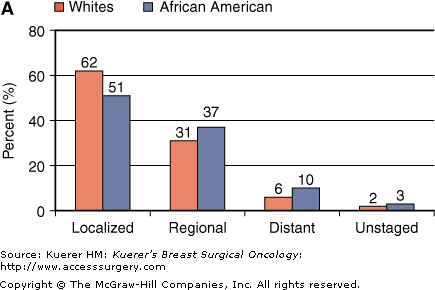
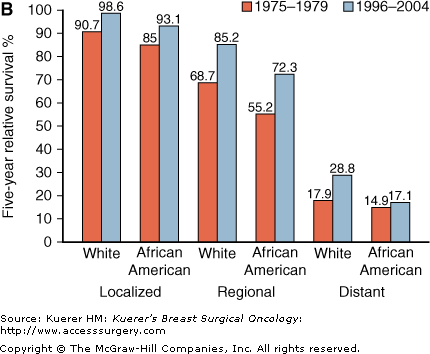
A. Distribution of breast cancer stage at diagnosis in African-American and white women, United States, 2000-2005. B. Five-year relative survival for African-American and white women by breast cancer stage, United States, 1975-1979 and 1996-2004.4(Data from Ries L, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2005. Bethesda: National Cancer Institute; 2008.)
Early age at menarche is a well-established risk factor for breast cancer in both premenopausal and postmenopausal women, with a reduction in risk of 5% to 10% for each year delay in age at menarche.9 Inaccurate recall of menarcheal age, especially in older women, may underestimate the strength of this association. Several mechanisms may explain the protective effects of late menarche. Early menarche may be associated with more rapid onset of regular, ovulatory menstrual cycles and hence longer duration of lifetime exposure to endogenous hormones.10 Estrogen levels are higher several years after menarche and remain so throughout the reproductive years in women with early menarche.11,12
Parous women have lower risk of breast cancer compared with nulliparous women, but the relationship between pregnancy and breast cancer is complex.10 Risk initially increases after the first pregnancy, then decreases after 10 years, and this protective effect is durable.13 In the long run, the protective effect outweighs the transient adverse effect. The short-term increased risk is thought to be due to significant elevated hormonal levels and rapid proliferation of breast epithelial cells, whereas the mechanisms for the long-term protection involves epithelial cell differentiation, which takes place largely after the first full-term pregnancy.14 Differentiated cells may be less sensitive to carcinogens due to longer cell cycles, allowing more time for DNA repair in the G1 phase.14 Additional births further reduce breast cancer risk though the effect is moderate. According to the collaborative reanalysis of 47 studies, each birth reduces the relative risk of breast cancer by 7%.15
Women with first full-term pregnancy after 35 years of age had 40% to 60% higher risk than those who had a first child before 20 years of age.10,16,17 However, more recent studies showed the effect was weaker,18 or did not exist for African Americans and indigenous Africans.18,19 For women who have later pregnancy, the transient postpregnancy risk increase mentioned earlier is more likely to manifest in epidemiologic studies, as age at delivery is closer to age at cancer diagnosis for these women. In addition, age at first birth is a less important factor among women with multiple children.19,20
Whether spontaneous and induced abortion are breast cancer risk factors is controversial.10 A meta-analysis documented an overall relative risk of 1.3 for induced abortion and no increased risk for spontaneous abortion, although there was a significant heterogeneity across studies.21 However, two large cohort studies in Denmark and the United States demonstrated that neither spontaneous nor induced abortion was associated with breast cancer (relative risk ≈ 1.0).22,23 A recent pooled analysis also concluded that abortions do not affect women’s risk of developing breast cancer.24 Furthermore, recent studies conducted in China, where the prevalence of induced abortion is high, found no link between induced abortion and breast cancer risk.25,26 Taken together, available evidences does not support induced abortion increasing breast cancer risk.
Growing epidemiologic evidence supports risk reduction with prolonged breastfeeding, although studies reported varied magnitude of this protective effect.10,15,27 In the pooled analysis of data from 47 epidemiologic studies, the relative risk of breast cancer decreased by 4.3% for every 12 months of breastfeeding.15 There is apparently a dose–response relationship, with increasing total duration of breastfeeding decreasing the risk.10,15,27 Inconsistent findings with respect to the comparison of ever versus never breastfeeding may be due to international variation in duration. In modern Western countries, where few women breastfed for more than 1 year, the protective effect was moderate, for example, a 22% risk reduction in premenopausal women was documented.28 In contrast, a risk reduction of more than 50% was observed for women with at least 5 years breastfeeding in populations of China,29 Mexico,30 and Nigeria.19 Several mechanisms have been postulated to explain the protective effect of breastfeeding.10,27 Breastfeeding delays the reestablishment of ovulation, thereby reducing lifetime endogenous hormones exposure. Breastfeeding may result in further terminal differentiation of the breast epithelium, thus making it less susceptible to carcinogenic stimuli.
As shown in Figure 4-3, the slope of increase in breast cancer incidence slows around menopause. It is well established that late age at menopause is associated with higher risk of breast cancer. For each year delay in age of menopause, the risk for breast cancer increases by 3%.10,31 Artificial menopause through bilateral oophorectomy also decreases breast cancer risk, while simple hysterectomy does not.10 In women with BRCA1 or BRCA2 mutations, bilateral oophorectomy reduces the risk of breast cancer by more than 50%.32
Many epidemiologic studies have evaluated the relationship between risk of breast cancer and oral contraceptives that contain estrogen and progestin. Generally, a positive but rather weak association has been seen. In a pooled analysis of 54 studies, current use of oral contraceptives was associated with 24% greater risk compared with never-users.33 This elevated risk disappeared after discontinuing: the relative risks were 1.16, 1.07, and 1.01, respectively, for women 1 to 4 years, 5 to 9 years, and 10 or more years after stopping use.33 Duration of use has no independent effect. As women usually use oral contraceptives in their second and third decade of life when the absolute risk of breast cancer is low, and the excess risk decreases after cessation of use, there are likely few breast cancer cases due to oral contraceptive use. A recent large case-control study also confirmed that former oral contraceptive use did not increase the risk later in life, when the breast cancer incidence of is higher.34
Health benefits and risk of postmenopausal hormones have been evaluated by numerous studies. In a pooled analysis of 51 epidemiologic studies, the current or recent use of postmenopausal hormones was associated with increased risk of breast cancer, with a dose–response relationship based on duration of use.35 The risk increased by 2.3% for each year of use and the relative risk was 1.35 for women who had used hormones for 5 or more years. This effect disappeared 5 years after discontinuing, regardless of duration of use. The effect of postmenopausal hormones was stronger in lean women than in obese women, a finding confirmed by later studies.36,37 Mounting evidence suggests that estrogen plus progestin increases breast cancer risk more than estrogen alone.31,35,37,38 Estrogen alone is associated with a relative risk of 1.3, while for estrogen plus progestin, relative risk ranges from 1.5 to 2.0 among several cohort studies.31,35,38 In the Women’s Health Initiative randomized trial, estrogen-only therapy did not increase risk, while estrogen plus progestin increased the risk by 26%.39,40
Attained height has been found to be positively associated with breast cancer in a large number of case-control and cohort studies.41-44 Numerous hypotheses related to nutritional influence on development have been proposed, including energy intake and growth during childhood (a hypothesis supported by variations in risk based on periods of nutritional deprivation as that which occurred in World War II), the interaction of nutrition with hormones during puberty, and the relationship of insulin-like growth factor with both height and breast cancer risk.
Excess body weight has been implicated as a risk factor, and has most often been examined via anthropometric measures such as body mass index (BMI—weight corrected for some function of total size). BMI has been convincingly correlated with breast cancer risk in numerous studies, although the relationship is often complex and involves additional modifying factors.36,44-51 In the Nurse’s Health Study involving more than 90,000 women, both high BMI and weight gain increased postmenopausal breast cancer risk.36 A pooled analysis of 7 cohort studies estimated excess breast cancer risk of 26% for postmenopausal obese versus lean women.44 Numerous other studies have found 1.25- to 2-fold or greater excess risk among postmenopausal obese women.45-50 Interestingly, obesity is associated with decreased breast cancer risk in premenopausal women in most studies.44,46,48,51
The predominant hypothesized mechanism in postmenopausal women implicates increased endogenous estrogen resulting from conversion of androgens by the aromatase enzyme in adipose fat.52 The association between increased circulating estrogen and breast cancer risk supports this concept,53,54 as does the apparent protective effect of obesity on breast cancer risk in premenopausal women, in whom high BMI is associated with decreased serum estradiol.55,56
Alcohol intake influences breast cancer risk, with a recent large meta-analysis of studies estimating relative risk increases of 32% for consumption of 35 to 44 g/day and 46% for 45 g/day or more, compared to women with no alcohol consumption.57
Tobacco use via smoking has not been consistently demonstrated to alter breast cancer risk, in part because this risk factor must be carefully evaluated in relation to other factors with which it may be correlated. In the above-referenced meta-analysis, the researchers identified a strong confounding influence of alcohol use on the effect of smoking, that when accounted for, resulted in no association remaining between smoking and breast cancer.57
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree