Primary tumor (T)
TX
Primary tumor cannot be assessed
T0
No evidence of primary tumor
Ta
Noninvasive papillary carcinoma
Tis
Carcinoma in situ: “flat tumor”
T1
Tumor invades subepithelial connective tissue
T2
Tumor invades muscularis propria
pT2a
Tumor invades superficial muscularis propria (inner half)
pT2b
Tumor invades deep muscularis propria (outer half)
T3
Tumor invades perivesical tissue
pT3a
Microscopically
pT3b
Macroscopically (extravesical mass)
T4
Tumor invades any of the following: prostatic stroma, seminal vesicles, uterus, vagina, pelvic wall, abdominal wall
T4a
Tumor invades prostatic stroma, uterus, vagina
T4b
Tumor invades pelvic wall, abdominal wall
Regional lymph nodes (N)
NX
Lymph nodes cannot be assessed
N0
No lymph node metastasis
N1
Single regional lymph node metastasis in the true pelvis (hypogastric, obturator, external iliac, or presacral lymph node)
N2
Multiple regional lymph node metastasis in the true pelvis (hypogastric, obturator, external iliac, or presacral lymph node metastasis)
N3
Lymph node metastasis to the common iliac lymph nodes
Distant metastasis (M)
MO
No distant metastasis
M1
Distant metastasis
27.1.5 Treatment
27.1.5.1 Non-Muscle-Invasive Bladder Cancer (NMIBC)
Transurethral Resection of Bladder Tumor (TURBT)
The gold standard management of NMIBC is a TURBT, aiming to remove all visible lesions. Detailed guidance on best practice is provided by many bodies including the European Association of Urology (EAU) and American Urological Association (AUA) [6, 7]. Many studies have evaluated the benefits of using Photodynamic Diagnosis (PDD) during which either 5-aminolevulinic acid (ALA) or hexaminolevulinic acid (HAL) are instilled into the bladder before cystoscopy is performed using ultraviolet light. Results are mixed with some studies finding no improvement in recurrence rates [8, 9] and others reporting improvements [10–12]. Currently the use of PDD is not routinely included in treatment algorithms but is used in many centers [6].
Risk Stratification/Surveillance
Patients with Ta and T1 tumors can be divided into low-, intermediate-, and high-risk groups using the EORTC scoring system [13]. The factors included are number of tumors, size of tumors, prior recurrence rate, T category in TNM staging, presence of concurrent carcinoma in situ and tumor grade.
Stratification into low, intermediate, and high risks can be used to identify which patients may benefit most from maintenance treatments. Low-risk tumors may only require one immediate post-operative instillation of chemotherapy, whereas those with intermediate risk are recommended to receive a year of maintenance treatment. This can be with either intravesical Bacillus Calmette–Guerin (BCG) or chemotherapy. Patients with high-risk tumors are recommended to continue BCG instillations for up to 3 years.
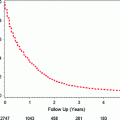
This risk stratification also helps determine which surveillance schedule is recommended, although no clear consensus exists about the precise follow-up schedule. The EAU guidelines suggest patients with low-risk tumors undergo cystoscopy at 3 months, 9 months and then annually for 5 years. Patients with high-risk tumors should undergo both cystoscopy and urine cytology at 3 months, then 3 monthly for 2 years, 6 monthly for 5 years, and annual cystoscopy thereafter. Patients with intermediate-risk tumors should have a surveillance schedule in between that for low- and high-risk patients [6].
Evidence for the use of both immunotherapy and chemotherapy exists in NMIBC.
Adjuvant Intravesical Bacillus Calmette–Guerin (BCG) Immunotherapy
Intravesical instillations using BCG have been shown to reduce recurrence rates compared to TURBT alone [14] and compared to intravesical chemotherapy [15, 16]. No clear guidance for dosing, timing, and durations are published, although there is consensus that it should be given on a maintenance schedule and not as a single post-operative instillation, unlike chemotherapy which can in some cases be given as a single treatment [16, 17]. The AUA advocates the use of the SWOG regimen, a 6-week induction course of BCG followed by a 3-week maintenance course at 3, 6, 12, 18, 24, 30, and 36 months [7].
Adjuvant Intravesical Chemotherapy
Adjuvant treatment with intravesical chemotherapy using mitomycin C (MMC), epirubicin, thiopeta, and doxorubicin are all used. However, as a result of limited availability of the other drugs, MMC is the most widely used. Sylvester et al. performed a meta-analysis of a single immediate post-operative instillation, including 7 randomized trials using MMC, thiopeta, and epirubicin, with recurrence data on 1476 patients and showed a significant decrease in the risk of recurrence with an odds ratio (OR) 0.61(p < 0.0001) [18]. The benefits of further instillations remain unclear. However, Sylvester et al. also performed a meta-analysis examining this and concluded that they may be beneficial in higher risk patients [19]. However, no clear guidance on the exact dosing, timing, and duration of ongoing instillations has been published.
Radical Cystectomy
In some cases of NMIBC radical cystectomy is indicated. These include all patients who have or develop BCG refractory disease. It should also be considered as a potential treatment in those with multiple, larger tumors, concurrent CIS, disease in the prostatic urethra, and micropapillary histology.
27.1.5.2 Muscle-Invasive Bladder Cancer
Radical Cystectomy + Lymph Node Dissection
Radical cystectomy with lymph node dissection is the gold standard treatment for MIBC if the patient is fit for surgery. Lymph node dissection can be either standard (regional bilateral node dissection), extended (to aortic bifurcation), or super-extended (to inferior mesenteric artery). Extended lymph node dissection has been shown in a number of retrospective studies to be beneficial [20, 21], but currently no clear recommendation for its use exists, pending the results of randomized controlled trials [22].
Neoadjuvant/Adjuvant Chemotherapy
Classically the 5-year survival rate, following radical cystectomy, is approximately 50 % [23] and so the benefits of adjuvant treatment have been investigated. Neoadjuvant chemotherapy, consisting of a cisplatin based regimen, has been shown to result in a 5 % improvement in overall survival in a number of meta-analyses for stage II and III disease [24–26]. The benefits of adjuvant chemotherapy, however, are not as well established, although a recent meta-analysis of nine randomized controlled trials revealed an overall survival benefit with a pooled hazard ratio of 0.77 (95 % confidence interval (CI) 0.59–0.99; p = 0.049). It is only currently recommended in high-risk patients who have not received neoadjuvant treatment [4].
Multi-modality Bladder Preservation
This approach is appropriate if patients are not fit for radical surgery or express a personal preference for a bladder conserving approach. Treatment is a combination of TURBT, chemotherapy, and radiotherapy, with a tri-modal combination being the preferred treatment option. The addition of cisplatin based chemotherapy improves outcomes with both radical and adjuvant radiotherapy following TURBT [27]. Five-year survivals between 50 and 60 % have been demonstrated, making this a feasible approach for some patients [28, 29]. These patients must undergo vigorous follow-up and bladder surveillance, as by retaining their bladder they remain at risk of both recurrence and new bladder tumors.
27.1.5.3 Advanced/Metastatic
First Line Treatment
Currently chemotherapy combinations, containing cisplatin, form the basis of first line treatment in advanced or metastatic disease. Both Gemcitabine and Cisplatin (GC) and Methotrexate, Vinblastine, Adriamycin, and Cisplatin (MVAC) have shown prolonged median survival rates of 13.8 months and 14.8 months [30, 31]. GC is less toxic than MVAC and is therefore usually the regimen of choice [30]. It is estimated that up to 50 % patients with advanced or metastatic disease are not fit to receive cisplatin therapy, due to a variety of factors including poor renal function and performance status [32]. It is common practice to replace cisplatin with carboplatin in this situation and to use it in combination with gemcitabine [33].
Second Line Treatment
Patients progressing following first line chemotherapy have limited further treatment options. Vinflunine, a third generation vinca alkaloid, remains the only licensed treatment in the second line setting for MBC. However, despite phase III trial evidence [34] and the lack of other treatment options, vinflunine has not been widely adopted into clinical practice.
An alternate strategy if relapse or progression occurs greater than 6 months after first line chemotherapy is to rechallenge the patient with cisplatin based combination chemotherapy or enrolment in a clinical trial.
New Treatments
In recent years, a large number of cytotoxic and targeted therapies have been investigated in phase II and III clinical trials, although these studies have yet to result in any new licensed treatments. At present, however, immunotherapy is undergoing a renaissance in solid cancer treatment. In advanced bladder cancer, checkpoint inhibitors targeting programmed cell death (PD-1) and its primary ligand PD-L1 have demonstrated the most promising results in the last 30 years. Powles et al. reported results of an extended phase I trial of Atezolizumab (MPDL3280A) a PD-L-1 inhibitor which demonstrated significant activity in advanced bladder cancer. They showed it is the most active against tumors with PD-L1 positive tumor infiltrating immune cells with objective response rates 43 % (13/30 patients CI 26–63 %), but still demonstrated objective response rates of around 11 % (4/35 CI 4–26 %) in PD-L1 negative tumors [35]. Similarly promising results were reported by Plimack et al. using Pembrolizumab, an anti-PD1 antibody [36]. This led to the US FDA granting this class of agent ‘breakthrough drug’ status in 2014 and later stage clinical trials are currently ongoing.
Palliative Treatment
Both surgery and radiotherapy are used as palliative treatments to treat localized symptoms such as frank hematuria or pain. Skeletal related events can also be minimized for patients with bone metastases using intravenous bisphosphonates [37]. In the absence of multiple treatment options for patients with advanced or metastatic disease, best supportive care remains of great importance [38].
27.2 Descriptive Epidemiology
27.2.1 Introduction
Globally, 430,000 men and women are diagnosed with bladder cancer and 165,000 individuals die each year [1]. The epidemiology of bladder cancer shows geographical, sex and age variations in its incidence, as well as being an example of a cancer where environmental and occupational risk factors are important in its etiology. In this section, the descriptive epidemiology will be discussed followed by the known risk factors for bladder cancer.
27.2.2 Geography
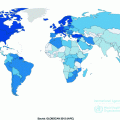
The incidence of bladder cancer varies greatly between the developed and developing world. The age-standardized incidence is 9.5 per 100,000 in more developed countries compared to 3.3 per 100,000 in less developed countries. The highest incidence is seen in Europe, North America, North Africa, and the Middle East. Some of these geographical differences in incidence can be explained by different registration practices for low-grade NMIBC which may significantly increase the recorded incidence. However, many studies have demonstrated that a migrant’s risk of developing bladder cancer approximates to that of their host country, suggesting different environmental factors are also important [39].
27.2.3 Sex
Bladder cancer incidence across the world is higher in males than females, with global rates of 9.0 per 100,000 versus 2.2 per 100,000. The sex difference in incidence tends to mirror that seen in lung cancer on a country-by-country basis, suggesting that the difference in smoking habits between the sexes is largely responsible for the differences rather than any hormonal or genetic influences [1].
27.2.4 Trends Over Time
The number of bladder cancer cases have risen over recent decades, with increases as large as 50 % in North America recorded between 1985 and 2005 [40]. These differences may reflect change in practice, with increasing registrations of lower grade tumors, rather than a true increase in numbers. Mortality rates in localized disease were static in the United States (U.S.) between 1973 and 2009, whereas they increased in metastatic disease by an estimated annual percentage increase of 1 % [41].
27.2.5 Age
Bladder cancer is a disease of aging, with the incidence gradually increasing with age in their 30s and 40s, before a sharp rise in both sexes after the age of 50. Most patients will not die from bladder cancer but will experience multiple recurrences. As a result, after prostate cancer older men with bladder cancer have the highest prevalence rates [1].
27.3 Risk Factors
27.3.1 Smoking
The most important risk factor for bladder cancer in the Western world is smoking and this association has been widely studied. There are over 60 carcinogens known to be in cigarettes, although the ones which are individually responsible for the increased risk of bladder cancer are not fully established [42]. The tobacco constituent 4-aminobiphenyl (4-ABP) however, is a well-established risk factor for bladder cancer. It is known to cause chromosomal instability in human cells [43]. It has also been shown that smokers of blond tobacco are at lower risk of bladder cancer than smokers of black tobacco, which is richer in 4-ABP [44].
One of the largest studies of the association between smoking and bladder cancer was the National Institutes of Health-AARP Diet and Health Study Cohort which included 280,000 men and 186,000 women who were followed from 1995 to 2006. The hazard ratio (95 % CI) for bladder cancer in current smokers compared to non-smokers was 4.1 (95 % CI 3.7–4.5) and remained elevated in ex-smokers (HR 2.2, 95 % CI 2.0–2.4). The authors estimated the population attributable risk of cigarette smoking to be 50 % in men and 52 % in women [45]. A meta-analysis including 11 case-control studies looking at ex-smokers and risk of bladder cancer showed a dose relationship with the number of cigarettes smoked per day and risk, although this reached a plateau at 15–20 cigarettes/day. This may be because of inaccurate recall when higher numbers of cigarettes are smoked per day or could be reflecting saturation in the biological mechanisms of carcinogenesis. They also reported a 30 % decrease in risk following cessation at 1–4 years and a 60 % reduction in risk at 25 years. But even at 25 years the risk did not return to that of never smokers [46].
The relationship between cigar and pipe smoking is not as well defined. One study looking particularly at cigar smoking failed to show any significant increased risk in all cigar smokers (Relative risk (RR) 1.0, 95 % CI 0.4–2.3), but did show an increased risk in a subset whom inhaled smoke (RR 3.6, 95 % CI 1.3–9.9) [47]. Therefore, both the method and type of smoking appear to be important in calculating the risk of developing bladder cancer.
Environmental tobacco smoke, also known as secondhand smoking, has been shown to increase risk of bladder cancer in lifelong non-smoking females, but not in non-smoking males. In a case-control study by Jiang et al. approximately twofold increased risks were seen among women living with a spouse or domestic partner who smoked for greater or equal to 10 years or who had a co-worker who smoked in an indoor environment for greater or equal to 10 years [48]. This may, in part be explained by higher quantities of the tobacco constituent 4-ABP in secondhand smoke [49].
27.3.2 Occupational Exposures
The association between occupational exposures and increased risk of bladder cancer was first described as early as 1895. The results of the first large occupational epidemiological study was published in 1954 [50] and examined the exposure of workman in the dye industry to aniline, benzidine, alpha-naphthylamine, and beta-naphthylamine. Occupations using these chemicals such as painters, mechanics, textile and metal workers have been shown to be at increased risk of bladder cancer. A RR as high as 40-fold has been reported for certain occupations but the intervention of health and safety executives, and the resulting reduction in exposure has contributed to a decrease in the incidence of occupational related bladder cancer in the developed world [51]. For a full list of known carcinogens please see Table 27.2.
Table 27.2
List of agents known to cause urinary bladder cancer
Carcinogenic agents with sufficient evidence in humans | Agents with limited evidence in humans |
|---|---|
Aluminum production 4-Aminobiphenyl Arsenic and inorganic arsenic compounds Auramine production Benzidine Chlornaphazine Cyclophosphamide Magenta production 2-Naphthylamine Painting Rubber production industry Schistosoma haematobium Tobacco smoking ortho-Toluidine X-radiation, gamma-radiation | 4-Chloro-ortho-toluidine Coal-tar pitch Coffee Dry cleaning Engine exhaust, diesel Hairdressers and barbers, occupational exposure Pioglitazone Printing processes Soot Textile manufacturing Tetrachloroethylene |