Abstract
Industrial chemical production has increased over the last few decades. In that same time frame, a number of adverse reproductive and health conditions have increased, including infertility, early puberty, obesity, gestational diabetes, preterm birth, children’s neurodevelopmental disorders, and noncommunicable chronic diseases such as cancer, cardiovascular disease, chronic respiratory disease, and diabetes. Basic science, animal model, and epidemiologic data indicate that specific environmental chemicals can interfere with hormone levels and action (referred to as endocrine disrupters) and thus may play an etiologic role in some of these conditions. These endocrine disrupters are found in pesticides (e.g., dichlorodiphenyl-trichloro-ethane [DDT], chlorpyrifos, atrazine, 2,4-dichlorophenoxyacetic acid [2.4D]), children’s toys and products (containing lead, phthalates, cadmium), materials that contact food (e.g., lining of plastic bottles or cans; bisphenol A, phthalates), building materials and electronics (e.g., brominated flame retardants), personal care products and intravenous tubing (e.g., phthalates), antimicrobials (e.g., triclosan), and textiles and clothing (e.g., perfluorochemicals). Endocrine disrupters can bind to hormone receptors, compete with endogenous hormones for receptor binding, stimulate or inhibit intracellular signaling mechanisms, alter gene expression, and affect epigenetic functions. Environmental chemical exposure effects are more severe during developmental periods and can be transgenerational. Given the impact on health broadly and reproductive health specifically, reproductive health providers need to understand the risks of environmental toxicants, their effects on reproductive capacity and outcomes, and ways to mitigate these effects, including advocacy for regulatory changes.
Keywords
Reproductive environmental health, pregnancy, prenatal, reproduction, environment, toxin, endocrine disruption, bisphenol A, phenol, phthalate, polyvinyl chloride, perfluorinated compound, pesticide, toxic chemicals, flame retardants
Introduction: Reproductive Health and the Environment
- ◆
Documented adverse trends in reproductive health cannot be explained by genetics alone.
- ◆
Exposure to environmental chemicals is ubiquitous and contributes to adverse trends in reproductive health and other human diseases.
Documented adverse trends in reproductive health in the United States include declines in fertility and in the age of onset of puberty and increases in rates of obesity, gestational diabetes, preterm birth, and children’s neurodevelopmental disorders, such as autism and attention-deficit/hyperactivity disorder (ADHD). These adverse trends are also reflected in a rise in noncommunicable diseases such as cancer, cardiovascular disease, chronic respiratory disease, and diabetes in high-, middle-, and low-income countries across the globe. Due to the timeframe of these changes, they cannot be explained by changes in genetic makeup alone. A possible explanation is exposure to environmental chemicals.
There is mounting scientific evidence of ubiquitous human exposure to environmental chemicals and associated adverse health impacts. In response to the evidence, reproductive health leaders and other scientists, clinicians, and their professional societies have called for timely action to prevent harm. This chapter summarizes reproductive and developmental health impacts of exposure to environmental chemicals, underlying mechanisms when known, and the role of clinicians and other health care professionals in prevention.
Definition
Reproductive environmental health addresses exposure to environmental contaminants, such as synthetic chemicals and metals (referred to collectively as environmental chemicals in this chapter) and their effects on reproductive health and development. Exposure to environmental chemicals can have an impact during all periods of human reproduction including fertility, conception, pregnancy, child and adolescent development, and adult health.
Reproductive Health Professional Society Engagement on Environmental Health
- ◆
Doctors in the United States and around the world have issued a call to action to prevent exposure to toxic environmental chemicals.
The contemporary roots of physicians’ organized engagement on reproductive environmental health issues date to 1957 when the American Academy of Pediatrics (AAP) became concerned about child health impacts of radioactive fallout from atmospheric testing of nuclear weapons; in the 1970s, the AAP broadened its focus to include the health consequences of exposure to environmental chemicals. The issue drew the concerted attention of reproductive health professionals in the 1990s with the publication of Generations at Risk: Reproductive Health and the Environment, which included compiled research linking environmental exposures to adverse reproductive health outcomes and intergenerational harm. In 2007, the University of California, San Francisco and the Collaborative on Health and the Environment convened the Summit on Environmental Challenges to Reproductive Health and Fertility , which brought together for the first time over 400 physicians, scientists, advocates, and policy makers to address reproductive environmental health science, clinical care approaches, and policy initiatives. In 2009, the Endocrine Society explicated the basic science of endocrine disrupting chemicals (EDCs) and the implications for human health and disease. In 2013, leading US and UK reproductive health professional societies formally recognized the opportunity to advance women’s health by championing the prevention of patient exposure to toxic environmental chemicals. In 2015, reproductive health professionals around the world issued a call to action on reproductive environmental health and established a structure to ensure clinical engagement to advance this topic among health practitioners and policy makers (see Recommended Online Resources ). Today, reproductive health professionals around the world have moved from awareness toward action on preventing exposure to environmental chemicals.
Key Scientific Concepts
- ◆
Preconception and prenatal exposure to environmental chemicals can impact the health of the pregnancy and the child, as well as adult health and the health of future generations.
- ◆
The fetus can be uniquely sensitive to even small amounts of environmental chemicals.
- ◆
Intergenerational harm can result from in utero exposure to exogenous chemicals.
- ◆
The placenta does not protect the fetus from many damaging chemicals, and fetal exposure can be higher than maternal exposure.
Several key scientific concepts underlie current understanding of reproductive environmental health.
Developmental Origins of Adult Health and Disease
The concept of developmental origins of adult health and disease describes links between the in utero environment, the external environment, an individual’s genes, and the propensity to develop disease or dysfunction later in life. The central nervous system, cardiovascular system, various endocrine systems, and the immune system are particularly vulnerable to adverse effects during development, and neurodevelopmental disorders and some cancers may well be initiated before birth or during early postnatal life.
The Fetus Can Be Uniquely Sensitive to Chemical Exposures: Thalidomide and Other Agents of Concern
The embryo, fetus, and developing human are highly vulnerable to exposure from even small amounts of environmental toxicants. This vulnerability is due to the unique and rapidly changing physiology, growth, and development of humans (i.e., high mitotic indices and cellular differentiation in developing organs; high metabolic rate; and an underdeveloped immune system, blood-brain barrier, and liver detoxifying mechanism).
A critical window of susceptibility occurs when exposures to environmental contaminants can disrupt or interfere with the physiology of a targeted cell, tissue, or organ ; exposures that occur during this time can lead to permanent and lifelong health effects and can be transmitted to subsequent generations. Sensitive windows of susceptibility can still permit the development of disease but with less intensity and severity as compared with those occurring in the critical window. Critical and sensitive windows occur during times of rapid development and cellular differentiation and proliferation such as those that occur during gametogenesis, embryogenesis, implantation, pregnancy, infancy, childhood, puberty, and lactation.
In the 1960s, the drug thalidomide was given to pregnant women to prevent morning sickness. While women who took the drug did not experience ill health effects, their children experienced a high rate of congenital limb and gastrointestinal malformations, particularly when their mother took the drug during the period of the 28th through the 42nd day after conception, which is the critical period of limb development. More than 10,000 children in 46 countries that approved the drug were born with deformities as a consequence of their mothers being prescribed the drug during pregnancy.
Intergenerational Harm Can Result From In Utero Exposure to Exogenous Chemicals: Diethylstilbestrol
Environmental chemical exposures can affect first generation (F0 [parental]) and second generation (F1 [offspring] and F2, germ cells of offspring) ( Fig. 19.1 ). To determine whether alterations are transmitted transgenerationally, the F3 generation must be studied.
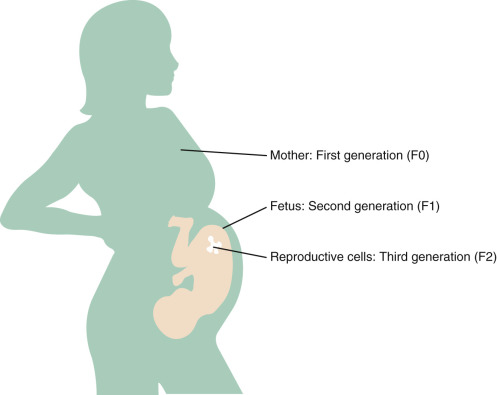
Adverse health impacts of in utero exposure to environmental chemicals may appear at birth, such as with thalidomide, and may also be delayed and/or manifest in future generations. This latter outcome is exemplified by the pharmaceutical diethylstilbestrol (DES), an estrogen that was prescribed in up to 10 million pregnancies from 1938 to 1971 to prevent miscarriage with no immediate adverse health impacts for the mother or child being observed. Reproductive tract abnormalities began to emerge in the daughters and sons of DES-exposed women only at puberty, and they continued to emerge 40 years after the index pregnancies and long after its use ended. Delayed health impacts of in utero exposure to DES include clear cell adenocarcinoma of the vagina and cervix, structural reproductive tract anomalies, infertility, poor pregnancy outcomes, and breast cancer among the daughters of women who took DES, and hypospadias among their sons and grandsons. This is a remarkable example of transgenerational inheritance, and mechanisms specifically underlying this process are unclear.
The Placenta Does Not Protect the Fetus From Many Damaging Environmental Chemicals: Methyl Mercury
Environmental chemicals can cross the placenta, and in some cases, such as with methyl mercury, they can bioaccumulate such that the fetal exposure is higher than maternal exposure. In the 1950s, waste discharge from a chemical factory in Japan polluted Minamata Bay, a primary source of food for the local population. Pregnant women who consumed contaminated fish and shellfish from the Bay were exposed to methyl mercury and subsequently gave birth to children with severe neurologic deficits, comprising a syndrome named Minamata disease . This tragedy established that the placenta does not protect the fetus from exposure to exogenous chemicals. Subsequent research has documented that developmental and cognitive effects can occur in children exposed prenatally to mercury even at low doses that do not result in effects in the mother and that the adverse neurologic effects of methyl mercury exposure may also be delayed.
Human Exposure to Environmental Chemicals
- ◆
Every day, everyone, everywhere is exposed to industrial chemicals.
- ◆
Exposures and risks are inequitably and unequally distributed.
Synthetic Chemicals and Heavy Metals Are Ubiquitous in the Environment
Chemicals in the environment are manufactured for various uses (e.g., synthetic chemicals for consumer and industrial applications); they are also by-products of industrial and human activity, and they are released through mining (e.g., heavy metals such as lead and mercury). Tens of thousands of chemicals circulate in global commerce, with over 4800 chemicals imported or manufactured in excess of 1 million pounds each per year. In the United States alone, chemicals in commerce increased more than 15-fold over the past 70 years; in 2012, 9.5 trillion pounds of industrial chemicals were domestically manufactured and imported into the United States, which is the equivalent of 30,000 pounds for every US resident. The vast majority of chemicals in commerce have not been fully tested for reproductive, developmental, or other health effects.
The thousands of high-volume chemicals in commerce are ubiquitous in the environment, which poses a major challenge to establishing health impacts and implementing effective interventions. Presented in the following are classes of chemicals and metals illustrative of the most widespread environmental chemicals. Table 19.1 presents where these chemicals and other common chemicals are found and specific health outcomes associated with exposure.
- •
Persistent organic pollutants (POPs): These include chemicals such as polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) that do not break down in the environment. While certain POPs such as the pesticide dichlorodiphenyl-trichloro-ethane (DDT), its metabolite dichlorodiphenyldichloroethylene (DDE), and PCBs have been banned in the United States for decades, they are still found in virtually the entire population due to their ubiquitous presence in the environment and long half-life and lipid solubility.
- •
Pseudo-persistent compounds: These compounds can be metabolized and removed from the body, but because exposure is consistent, they are found in most human samples. Examples include phthalates, polycyclic aromatic hydrocarbons (PAHs), perfluorochemicals (PFCs), bisphenol A (BPA), and perchlorate.
- •
Metals : Metals commonly found in the environment include mercury, arsenic, and lead. Due to public policy initiatives that removed lead from gasoline, paint, food cans, and other products, blood levels of lead have dropped precipitously in the United States. However, the 2015 discovery of lead in Flint, Michigan’s drinking water reveals that there remain populations within the United States with high blood lead levels, which are often caused by exposure to lead-contaminated paint and lead in water pipes.
| Chemical | Exposure Sources and Pathways | Selected Health Impact (Reproduction, Poor Birth Outcome, Neurodevelopment, and Cancer) |
|---|---|---|
| PCBs | Used as industrial insulators and lubricants; banned in the 1970s, but persistent in the aquatic and terrestrial food chains, which results in exposure by ingestion. |
|
| PFAS | Widely used man-made organofluorine compounds with many diverse industrial and consumer product applications; examples are PFOS and PFOA, which are used in the manufacture of nonstick Teflon and other trademark cookware products and in food-contact packaging to provide grease, oil, and water resistance to plates, food containers, bags, and wraps that come into contact with food; persist in the environment; occupational exposure to workers and general population exposure by inhalation, ingestion, and dermal contact. |
|
| PBDEs | Flame retardants that persist and bioaccumulate in the environment; they are found in furniture, textiles, carpeting, electronics and plastics that are mixed into, but not bound to, foam or plastic. |
|
| Phenols | Examples are BPA, triclosan, and parabens. BPA: Chemical intermediate for polycarbonate plastic and resins; found in consumer products and packaging; exposure through inhalation, ingestion, and dermal absorption. |
|
| Triclosan: Synthetic chlorinated aromatic compound with antibacterial properties; used in many consumer products such as antibacterial soaps, deodorants, toothpastes, cosmetics, fabrics, plastics, and other products; exposure is through ingestion, dermal contact, and consumption of contaminated food and drinking water. |
| |
| Parabens: Most commonly used preservatives in cosmetic products, including makeup, moisturizers, hair care products, and shaving products; also used in foods and drugs; exposure through dermal absorption and ingestion. |
| |
| Phthalates | Synthetically derived; used in a variety of consumer goods such as medical devices, cleaning and building materials, personal care products, cosmetics, pharmaceuticals, food processing, and toys; exposure occurs through ingestion, inhalation, and dermal absorption. |
|
| Heavy metals | Cadmium: Used in batteries, pigments, metal coatings, and plastics; for nonsmoking public, exposures mainly occur through diet (shellfish, organ meats, grains such as rice and wheat, leafy vegetables, and some root crops such as potato, carrot, and celeriac); for smokers, exposure mainly occur through tobacco smoke. |
|
| Lead: Occupational exposure occurs in battery manufacturing/recycling, smelting, car repair, welding, soldering, firearm cleaning/shooting, stained-glass ornament/jewelry making; nonoccupational exposure occurs in older homes where lead based paints were used, in or on some toys/children’s jewelry, water pipes, imported ceramics/pottery, herbal remedies, traditional cosmetics, hair dyes, contaminated soil, toys, costume jewelry. |
| |
| Mercury: Coal-fired power plants are largest source in the United States; primary human exposure is by consumption of contaminated seafood. |
| |
| Perchlorate | Used to produce rocket fuel, fireworks, flares, and explosives and can also be present in bleach and in some fertilizers; primary pathway for exposure is through drinking water caused by contaminated runoff. |
|
| Pesticides | Applied in large quantities in agricultural, community, and household settings; in 2007, >1.1 billion pounds of active ingredients were used in the United States; it can be ingested, inhaled, and absorbed by the skin; pathways of exposure include food, water, air, dust, and soil. |
|
| Solvents | Liquids or gases that can dissolve or extract other substances; they are used in manufacturing, service industries such as dry cleaning and printing, and consumer products including stain removers, paint thinners, nail polish removers, and hobby/craft products; examples are benzene, gasoline, ethyl alcohol, methanol, phenol, styrene, toluene, trichloroethylene, and xylene; exposure occurs through inhalation, dermal absorption, and ingestion. |
|
Environmental Chemicals in Pregnant Women
Environmental chemicals permeate the air, water, food, and consumer products; consequently, exposure to environmental chemicals among pregnant women in the United States and around the world is ubiquitous. A report by the US National Cancer Institute concluded that “to a disturbing extent babies are born ‘pre-polluted’.” In population-based surveys of pregnant women in the United States, specific PCBs, organochlorine pesticides, PFCs, phenols, PBDEs, phthalates, PAHs, and perchlorate were detected in 99% to 100% of pregnant women. Many chemicals were measured at levels similar to the levels encountered in epidemiologic studies that demonstrated adverse reproductive and developmental outcomes. Furthermore, virtually all women were exposed to multiple chemicals. Exposure to multiple chemicals that impact the same health endpoint (e.g., brain development) can result in a greater risk than by exposure to a single chemical alone.
Fetal Exposure to Environmental Chemicals
As exemplified by the Minamata disaster described previously, placental transfer of chemicals is now well established. Fetal exposure varies depending on the chemical structure and composition, with some chemicals having higher or lower affinity for transplacental transfer. For example, measured concentrations of 87 environmental pollutants in mother-child samples demonstrated that all substances found in the mothers were also present in fetal tissues and cord blood; however, the degree of transplacental passage and correlation with maternal levels varied depending on chemical structure, lipid solubility, and molecular weight. Recent research suggests that fetal exposure exceeds maternal exposures not just for methyl mercury but also for many other environmental chemicals.
Exposure and Risks Are Inequitably and Unequally Distributed
Exposure to toxic environmental chemicals and associated adverse health outcomes are inequitably and unequally distributed among people, communities, and countries. Some of these differences in vulnerabilities and risk relate to age, sex, genes, underlying health status, and coexposure to other environmental stressors. Other vulnerabilities are related to socioeconomic status, occupation, and/or racial discrimination and other social factors that increase stress. For example, people with low incomes bear a disproportionate burden of morbidity and mortality, loss of family income and productivity, and environmental degradation related to environmental exposures ; indigenous peoples in Canada, the United States, and other countries incur a higher burden of toxic exposures and resulting adverse health outcomes. Many of these factors can predispose the population to be more sensitive to the effects of chemical exposures and multiple vulnerabilities increasing that sensitivity. Thus environmental chemicals may be an important factor in health disparities. Furthermore, as these differences mediate risk, the National Academy of Sciences has recommended that in the absence of evidence to the contrary, any level of exposure should be assumed to be potentially harmful (i.e., there is no assumption of a “safe dose”).
Mechanisms
- ◆
Exposure to endocrine disrupting chemicals can adversely impact reproductive success by interfering with any aspect of hormone action.
- ◆
Epigenetic modifications affect gene expression, cell and tissue function, and disease risk.
- ◆
Through epigenetic mechanisms, environmental chemicals, including but not limited to endocrine disrupting chemicals, can impact reproductive success and other aspects of healthy human development across generations.
Endocrine Disruption
EDCs can modify multiple biological processes by interfering with any aspect of hormone action, and thus these chemicals have the potential to affect steroid hormone-dependent human reproductive tract development and adult reproductive function. Exposure to DES and other chemicals (see later) are a testimony to these interferences. In addition to being reproductive “disrupters,” EDCs have recently been implicated as thyroid disrupters, neurodevelopmental disrupters, obesogens, and diabetogens, which directly and indirectly affect reproductive success.
The question arises, through what mechanisms do EDCs affect such a diverse set of functions and processes? It is not surprising that they are mediated by multiple mechanisms. For example, specific hydrophobic EDCs in cigarette smoke, plasticizers, pesticides, cosmetics, and dietary components interact with estrogen receptors (ER) α and β, bind to estrogen response elements (EREs), and activate or repress gene expression via genomic pathways. In addition, they can evoke rapid responses involving membrane-associated ERs and affect nongenomic pathways, including kinase signaling cascades, as well as binding and blocking androgen action at the androgen receptor (AR). Some EDCs can also bind to the aryl hydrocarbon receptor (AHR), associate with AHR nuclear translocator (ARNT), and bind to dioxin response elements (DREs). EDCs can also change cellular processes by activating ion channels, inducing proinflammatory cytokines and chemokines, promoting oxidative stress, and altering cell proliferation and differentiation. In addition, EDCs can act via nonsteroid hormone receptors (e.g., GPR30, peroxisome proliferator-activated receptor [PPAR], and the thyroid receptor ThR) and affect enzyme activities and organ patterning.
Epigenetic Mechanisms
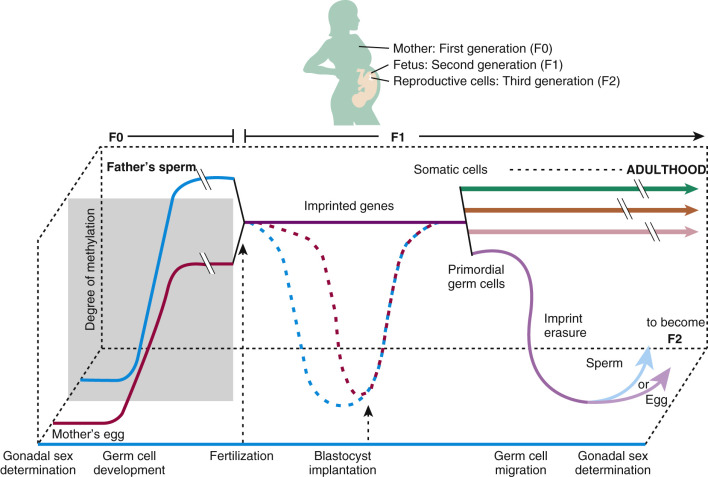
Increasing evidence suggests that epigenetic mechanisms underlie tissue and organ dysfunction due to some EDCs, including DNA methylation, histone modifications, and microRNA (miRNA) expression and subsequent gene expression. In vitro studies with human and rodent cell lines and in vivo data in rodents and humans reveal effects of environmental chemicals, including EDCs, on global and gene promoter-specific DNA methylation. For example, arsenic and cadmium as well as DES and benzene affect genome-wide DNA methylation and promoter methylation regulating p53, p15 , and the Agouti gene expression. Maternal smoking affects genome-wide promoter regions of the human placenta (14,000 genes), as well as specific genes (e.g., LINE1 and AluYb8 ) in the placenta. Histone acetylation, dimethylation, trimethylation, and monoubiquitination modifications have been observed with heavy metals, and multiple miRNAs, including tumor suppressing and oncogenic miRNAs and placental miRNA changes associated with maternal smoking. These modifications in turn affect gene expression, cell and tissue function, and disease risk. Importantly, the impact of EDCs can be significant for reproductive success across generations, especially considering the methylation/demethylation changes that occur normally during gametogenesis and embryogenesis ( Fig. 19.2 ). Environmental modifications of gene expression can affect embryonic imprinting, cellular differentiation, and phenotypic expression that can have F0, F1, F2, and F3 generational impacts.
Reproductive Health Outcomes Linked to Environmental Chemical Exposures
- ◆
Prenatal exposure to environmental chemicals commonly found in our air, water, food, and consumer products are linked to myriad adverse reproductive health outcomes.
- ◆
Exposure to endocrine disrupting chemicals may significantly impact both female and male reproductive capacity.
- ◆
Adverse reproductive health outcomes linked to exposure to environmental chemicals include fertility and fecundity, pregnancy, and neonatal and child health outcomes.
The literature demonstrating links between environmental chemical exposures and adverse reproductive outcomes has grown substantially over the past several decades. Prenatal exposure to environmental chemicals has been linked to a diverse range of health impacts across the lifespan of individuals, including, but not limited to, harm to healthy human reproduction, poor birth outcomes, neurodevelopmental problems, and cancer. Table 19.1 presents examples of adverse health impacts of prenatal exposure to chemicals found commonly in our air, water, food, and/or consumer products.
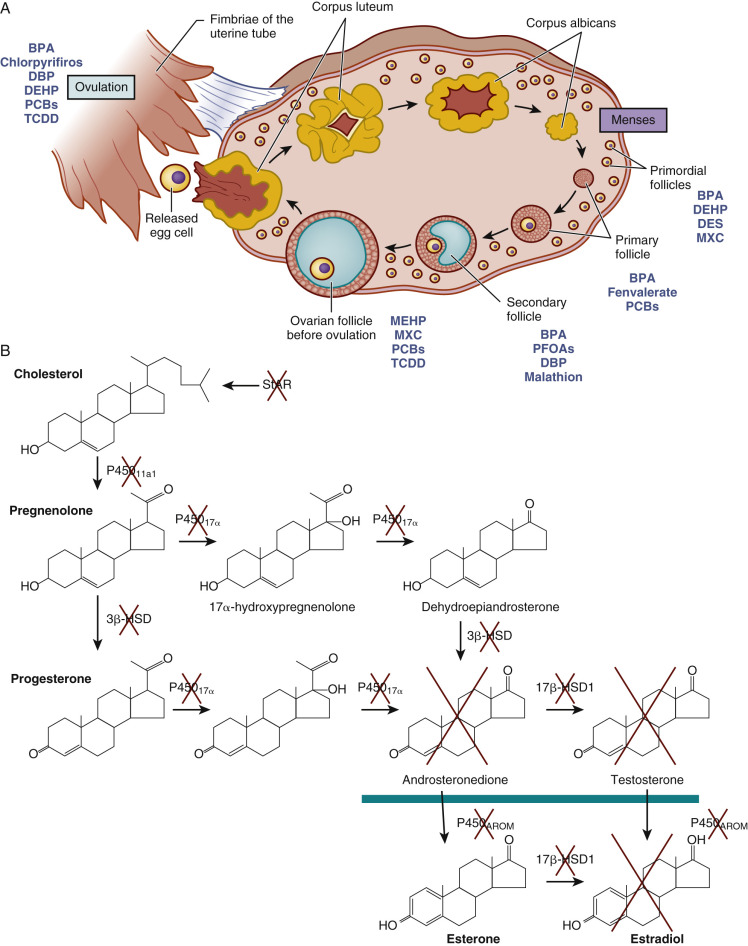
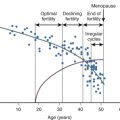
Studies in mice, rats, hamsters, lamb, sheep, and humans reveal that EDCs may significantly impact female reproductive capacity, including ovarian steroidogenesis, oocyte quality, menopause timing, age of puberty, and reproductive disorders (see Chapter 8 ). Fig. 19.3 pictorially demonstrates disruption at nearly every stage of ovarian follicle development and each stage of steroidogenesis. Similar disruption in the male occurs, for example, with prenatal exposure to some EDCs (mostly antiandrogens, estrogens, and dioxins) and increased risk of cryptorchidism, hypospadias, abnormal fetal and adult Leydig cell and Sertoli cell steroidogenesis, sperm quality, and testicular (germ cell) cancer. Fig. 19.4 illustrates the testicular dysgenesis syndrome (TDS) proposed by Skakebaak (2003) in which spermatogenesis, undescended testis, and hypospadias as well as testicular (germ cell) cancer are part of a spectrum of male reproductive tract dysfunction due in part to EDCs. These syndromes range in severity from mild, to medium, to severe TDS, and they can have transgenerational impact.