Integrins
Integrins are transmembrane
αβ heterodimers that bind many extracellular matrix proteins and certain immunoglobulin-like adhesion molecules to other cells.
9,10 Most integrins require conformational activation to support binding. The mechanisms of integrin activation have been studied in detail for
αVβ3,11 an integrin also expressed on monocytes, and lymphocyte function-associated antigen 1(LFA-1),
12 an integrin highly expressed on monocytes and other leukocytes. Activation of
αxβ213 and
α4β1 integrins
14 has also been studied. Both are expressed on blood monocytes at low and high levels, respectively. Integrin activation is probably initiated by the binding of the intracellular cytoskeletal adaptor molecules talin and kindlin-3 to the integrin
β chains,
15,16 which causes extension of the extracellular domain that exposes the ligand-binding site and greatly increases the on-rate of ligand binding (
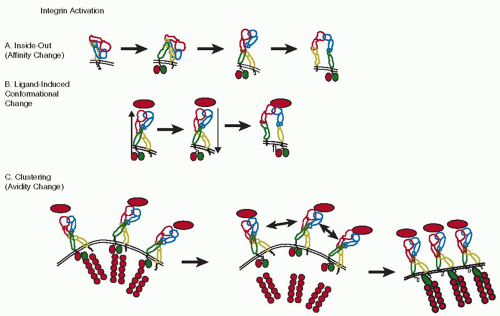
FIGURE 46.1A). This process is called inside-out signaling, because the change in the extracellular domain of the integrin is brought about by intracellular processes. The mechanism of activation-induced conformational change is thought to apply fairly generally to many, if not all integrins.
10 However, the regulation of affinity by reducing the dissociation of the ligand from the integrin is much less clear. Although conformational changes in the ligandbinding domain have been reported,
17 it is not clear whether these changes are directly linked to integrin extension or could be regulated separately.
14Integrins may first undergo some initial conformational change (probably extension) through inside-out signaling, followed by ligand binding, which then causes outside-in signaling that leads to full activation and strong binding
10,18,19 (
FIGURE 46.1B). However, not all experimental data are consistent with this model.
20 In addition, integrins can also rearrange in the plasma membrane to cluster and form patches, which results in enhanced binding (
FIGURE 46.1C). This rearrangement and clustering probably happens after ligand binding, results in increased binding of multivalent ligands, and is called avidity change. It does not result in increased affinity for the monovalent ligand.
21 Under
in vivo conditions, cell activation probably results in a combination of integrin affinity and avidity increase. Although this can be tested by looking at monovalent versus polyvalent ligand binding, the role of avidity and affinity regulation remains controversial.
10,18,21Of the 24 known integrins, mature blood monocytes express only a few. The most abundant monocyte integrin is
α4β1 integrin or very late antigen-4 (VLA-4) (CD49d/CD29). It is composed of a 150 kDa
α4 chain that can undergo proteolytic cleavage and a noncovalently associated 130 kDa
β1 chain.
α4β1 integrin is preferentially expressed on cell surface projections that are often called microvilli, but resemble ridges rather than true villous processes.
22 This position is believed to facilitate the interaction of
α4β1 integrin with its ligands under conditions of flow. The most important ligands for
α4β1 integrin include
the vascular cell adhesion molecule 1 (VCAM-1) on endothelial and other cells (
Table 46.2) and the heparin-binding region of alternatively spliced fibronectin expressed in the extracellular matrix and on the luminal surface of inflamed endothelial cells.
23 Like other integrins,
α4β1 integrin can probably undergo conformational activation.
14,24,25 This process of affinity regulation can be triggered by monocyte activation, for example, through chemokines. Gene-targeted mice lacking either
α426 or
β127 are not viable. Blocking
α4β1 with a monoclonal antibody or a peptide based on the fibronectin sequence ILDV reduces atherosclerosis in mice,
28 suggesting that
α4β1 is important in monocyte recruitment to atherosclerotic lesions.
The integrin
αMβ2 (CD11b/CD18) is also known as Macrophage-1 or Mac-1. Mac-1 antibodies were some of the earliest monocyte-macrophage specific antibodies described. Although Mac-1 is also expressed on neutrophils, most of its function seems to be related to monocyte-macrophages. First, Mac-1 participates in monocyte adhesion to various substrates including endothelial cells. Second, Mac-1 is an important receptor for the complement and is also known as complement receptor 3 (CR3). Mac-1 binds complement C3bi and is critically involved in phagocytosis of complement-coated bacteria and particles. Mac-1 engagement promotes a proinflammatory response, including a respiratory burst with vigorous oxygen radical production, actin polymerization, induction of nitric oxide synthase, and shape change. Interestingly, under flow conditions such as those achieved in flow chambers
in vitro, or isolated perfused vessels
ex vivo, Mac-1 does not appear to contribute to monocyte adhesion to endothelial cells.
29 Mac-1 has been shown to lower the rolling velocity of neutrophils,
30 but the role of Mac-1 in monocyte rolling has not been studied. Mice lacking Mac-1, prepared by targeting the gene for
αM, are viable, fertile, and healthy under vivarium conditions.
31 There is no evidence that these mice are protected from atherosclerosis, but their response to vascular injury is blunted.
32 Like all
β2 integrins, Mac-1 has an I-domain with homology to the von Willebrand factor (vWF) A-domain inserted in its
α subunit, which contains the ligand-binding site. Mac-1 binds many other ligands
including fibrinogen and coagulation factor × (
Table 46.2). Mac-1 is thought to be involved in assembling prothrombinase on the monocyte surface and may be able to support platelet binding to monocyte through a fibrinogen bridge between
αIIbβ3 on platelets and Mac-1 on monocytes.
33 Although Mac-1 deficient mice have no obvious defect in hemostasis, Mac-1 could participate in monocyte activation and the delivery of tissue factor to sites of thrombosis.
34 Human, but not mouse monocytes also express a closely related integrin,
αxβ2, which is also a complement receptor, alternatively known as CR4. Abundant
αx expression is found on dendritic cells. Like Mac-1,
αxβ2 binds the intercellular adhesion molecule 1 (ICAM-1), C3bi, and denatured proteins,
35 but in addition VCAM-1 also.
36The LFA-1, or
αLβ2 integrin (CD11a/CD18) is expressed on all leukocytes including monocytes. While LFA-1 is responsible for lymphocyte arrest, the sudden stopping of rolling cells upon activation
37 and participation in neutrophil arrest under flow, little is known about its function in monocytes. LFA-1 binds to cell surface immunoglobulins including InterCellular Adhesion Molecules ICAM-1 and-2, and has no known extracellular matrix ligands. Mice lacking LFA-1 were prepared by targeting the gene for
αL38 and are viable, healthy, and fertile under vivarium conditions. There are no reports of these mice having altered thrombosis, hemostasis, or atherosclerosis. Like the other
β2 integrins, LFA-1 has an I-domain and undergoes extensive conformational changes of the extracellular domain upon activation.
12The integrin
αVβ3 is expressed on blood monocytes at a low copy number. Its expression increases upon differentiation to osteoclast-like cells. This integrin was initially called leukocyte response integrin
39 because it participates in inducing the respiratory burst associated with NADPH oxidase activation and oxygen free radical production in neutrophils. Ligands for
αVβ3 integrin include vitronectin, entactin, and possibly the immunoglobulin-like adhesion molecule L1. Gene-targeted mice lacking
αV are not viable, whereas mice lacking
β3 have a defect in both
αVβ3 on monocytes, neutrophils, and proliferating endothelial cells, and
αIIbβ3 on platelets, which share the common
β3 subunit. The phenotype of these mice is dominated by the platelet defect (Glanzmann thrombasthenia-like), and these mice also have osteosclerosis, suggesting defective osteoclast function.
40 αVβ5 integrin is also a vitronectin receptor. Monocytes express
α2β1 integrin, a collagen receptor, at the mRNA and protein levels.
α5β1, a fibronectin receptor,
α10β1, a collagen receptor, and
αEβ7, a receptor for E-cadherin, are found at the message level, but functional data have not been published.
Immunoglobulins
Blood monocytes express many immunoglobulin-like molecules. Of importance for this chapter is ICAM-1, because it supports homotypic aggregation of monocytes via LFA-1 and Mac-1 and because it can bind fibrinogen.
41 Mice with hypomorphic mutations in the ICAM-1 gene or lacking ICAM-1 entirely have no overt defect in hemostasis or thrombosis, but are somewhat protected from atherosclerosis in the C57BL/6 and apolipoprotein E knockout models.
42 However, these mice also lack ICAM-1 on endothelial cells, smooth muscle cells, lymphocytes, and many other cells. ICAM-2 and ICAM-3 are found in monocytes at the message level, but have no known function in monocytes.
Platelet endothelial cell adhesion molecule 1(PECAM-1) (CD31) is a homotypic adhesion molecule expressed on blood monocytes and has an important role in transendothelial migration and in monocyte activation.
43,44 Monocyte PECAM-1 interacts with PECAM-1 on endothelial cells during transmigration. PECAM-1-deficient C57BL/6 mice have no apparent defect in leukocyte and monocyte transmigration, demonstrating that PECAM-1-independent pathways of transmigration exist.
45Other immunoglobulin-like molecules expressed on monocytes include major histocompatibility complex (MHC) class II, which is important in antigen presentation, but is not fully induced until monocytes differentiate to macrophages. CD83 is also expressed and has costimulatory functions in macrophages, but no known function in blood monocytes.
Selectins and their Ligands
L-selectin (CD62L) is expressed on “inflammatory” blood monocytes and most other leukocytes. Its most important function is in lymphocyte homing to secondary lymphatic organs,
46 but it is also involved in inflammation.
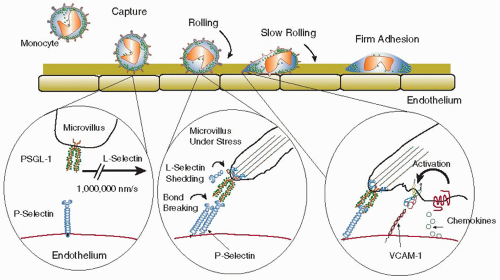
47 Like the other selectins, L-selectin can mediate leukocyte rolling, a passive motion of leukocytes down a vessel wall driven by the blood flow and the forces exerted on the loosely attached cell. During rolling, molecular bonds form at the leading edge and continually break at the trailing edge of the cell, allowing the leukocyte to stay in contact with the endothelium without actually stopping
48 (
FIGURE 46.2). Rolling is thought to serve to “scan for” inflammatory stimuli, and rolling cells can stop (arrest) in response to appropriate stimuli.
18,49 On neutrophils, L-selectin is expressed on the tips of microvilli
50 and can be rapidly shed upon cell activation by a protease-dependent mechanism involving tumor necrosis factor-α-converting enzyme (TACE) (ADAM-17).
51 Although L-selectin has been shown to support monocyte rolling on L-selectin ligands in flow chambers, it is not known whether L-selectin-mediated monocyte rolling on endothelial cells serves a physiologic function. Endothelial ligands for L-selectin known as peripheral node addressins are expressed in endothelial cells of lymphoid organs, but L-selectin ligands on endothelial cells outside lymphoid organs have not been identified. It is tempting to speculate that monocyte L-selectin may enhance monocyte recruitment to atherosclerotic lesions by nucleating monocyte-monocyte interactions through binding
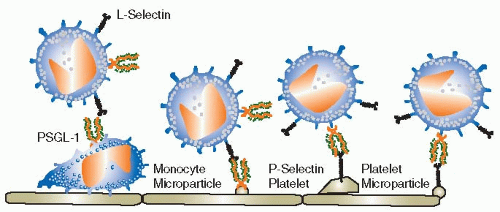
to P-selectin glycoprotein ligand-1 (PSGL-1, CD162, see below) in a process called secondary capture
52 (
FIGURE 46.3). Secondary capture is initiated when a vessel wall-adherent monocyte or monocyte-derived microparticle exposes PSGL-1 to other monocytes that pass by in the free stream. These cells can transiently bind to the adherent monocyte through L-selectin and then attach to the endothelium downstream from the nucleation site. While this process is certainly plausible and has been observed to occur in mouse aortas,
53 no direct evidence has been provided to support its importance in atherosclerosis, thrombosis, or hemostasis.
The most important selectin ligand on monocytes is PSGL-1, (CD162). PSGL-1 is somewhat of a misnomer, because PSGL-1 also binds L- and E-selectins with similar affinity as P-selectin.
54 All monocytes, like most leukocytes, express PSGL-1 protein on the cell surface, but not all cell surfaceexpressed PSGL-1 can bind selectins. PSGL-1 binding to selectins is regulated by a series of glycosyltransferases and sulfotransferases required to make it a functional selectin ligand (
Table 46.3). Blood monocytes express fucosyltransferase VII, core2
N-acetylglucosaminyltransferase-I, and at least one sialyltransferase. Therefore, PSGL-1 on monocytes is constitutively active and can bind all three selectins. PSGL-1 is a covalent homodimer and, like VLA-4 and L-selectin, is expressed on microvilli.
55Recently, PSGL-1 has been shown to be of key importance for the delivery of tissue factor to sites of thrombosis.
56,57 PSGL-1-expressing microparticles, presumably derived from monocytes, deliver tissue factor to sites of thrombosis in a PSGL-1 and P-selectin-dependent process. Apparently, the generation of these microparticles can be induced by soluble P-selectin
58 in a process that requires PSGL-1 expression. Taken together, PSGL-1 is one of the most important molecules connecting inflammation with hemostasis and thrombosis. PSGL-1 deficient mice show reduced inflammatory responses in many models and have a remarkable defect in tissue factor recruitment to sites of vascular injury and thrombosis.
56,57 The importance of PSGL-1 is underlined by epidemiologic evidence that humans expressing PSGL-1 alleles that encode shorter isoforms (fewer tandem repeats) are relatively protected from cardiovascular disease.
59 The shorter versions of PSGL-1 support less monocyte-platelet adhesion.
Recently, P-selectin was also found to be expressed on peritoneal macrophages
60 and in foam cells found in the neointima
formed after vascular injury in atherosclerotic mice.
61 There is no evidence for P-selectin expression on blood monocytes.