The development of microendoscopes for visualization of mammary ductal anatomy has progressed rapidly over the past 2 decades. The first published reports of mammary ductoscopy appeared in 1991 authored by Okazaki et al as well as Makita et al in which the endoscopy was performed with essentially a bare fiberoptic cord with no working channel for insufflation or aspiration.1,2 Subsequently, Susan Love published a small series of breast endoscopy in 9 patients with a diagnosis of ductal carcinoma in situ at the time of mastectomy.3 Again, using an early generation instrument, they encountered difficulties with insufflation of the ducts and navigation of ductal branches due to rigidity of the scope. As technology improved, a number of semiflexible microendoscopes emerged, which allowed insufflation via a working channel that was formed by a sheath that surrounded the fiberoptic core. Reports of the feasibility of visualization and navigation to the level of the terminal ductal lobular units were generated initially in human mastectomy specimens and subsequently in patients under local anesthesia.4,5 These technologic developments resulted in visualization of submillimeter intraductal lesions and access into the terminal human mammary ducts, which has spawned a new era of intraductal approaches to diagnosis and treatment of breast diseases.
The anatomy and pathophysiology of the human mammary ductal system is still an area of active research investigation. Murine transgenic models continue to provide insight into the checkpoints and proliferative mechanisms that are critical to development of the mature mammary ductal system. Interestingly, injection molds of human mastectomy specimens date back to the early anatomists such as Sir Astley Paston Cooper, who published his illustrations in the treatise On the Anatomy of the Breast in 1840. A modern counterpart to these anatomic studies has been published by JJ Going which elegantly details the fine arborization of ductal systems that are overlapping but not communicating (Fig. 58-1).6 This pattern of complex arborization has been confirmed in patients undergoing galactography as well. In total, these studies illustrate the anatomic challenges that face investigators who have plans to survey and sample the human mammary ductal system.
Figure 58-1
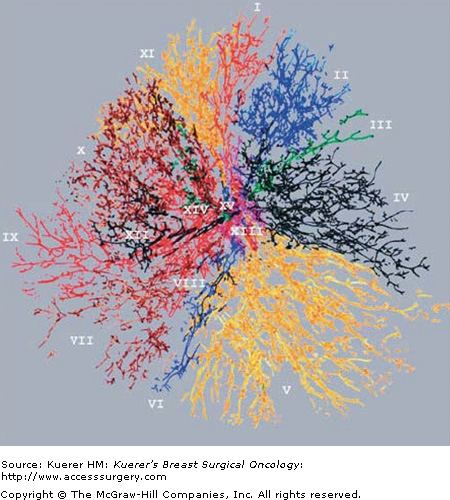
Reconstruction of human mammary ductal system. A human mastectomy specimen was injected with artificial materials of different colors to form a cast of the human mammary ductal system. Each color represents a different ductal system. Note the tremendous branching and the overlap of ductal systems which do not communicate. [Reproduced, with permission, from Going JJ, Moffat DF. Escaping from flatland: clinical and biological aspects of human mammary duct anatomy in three dimensions. J Pathol. 2004;203(1):538-544.]
Pathologic nipple discharge (PND) has been subjected to a wide variety of definitions that illustrate one source of variation in comparing patients enrolled in clinical trials at different institutions. One critical defining feature is to differentiate pathologic nipple discharge from physiologic nipple discharge. In the instance of physiologic nipple discharge the consensus is that this is due to hormonal or endocrine stimulation of the breast glandular tissue resulting in bilateral, multiduct discharge. By contrast, pathologic nipple discharge is easiest to diagnose when it is unilateral, uniductal, spontaneously elicited, and bloody. However, if pathologic nipple discharge is defined by the presence of intraductal pathology on subsequent duct excision (both benign and malignant), then there are instances where clear discharge, nonspontaneous discharge, bilateral discharge, and multiduct discharge can all be associated with pathologic findings.7 One such study, which carefully evaluated the characteristics of the nipple discharge (milky, sticky, purulent, bloody, etc), of 586 patients who underwent duct excision demonstrated benign findings in over 70% (intraductal papillomata 48%, and fibrocystic changes 33%). As a general rule, most authors agree that although intraductal papilloma is by far the most common etiology for PND, the character of the discharge cannot always predict the absence of malignancy.
Patients who present with pathologic nipple discharge undergo routine breast physical exam as well as mammography as part of their complete evaluation. Patients who have abnormal findings on any of these tests typically will then undergo either further imaging or biopsy of the imaged or palpable abnormality. In patients with PND who have no palpable breast mass or mammographic abnormality, a variety of diagnostic tests have been analyzed for sensitivity and specificity. Cytology of nipple discharge has notoriously yielded variable sensitivity and specificity, with individual studies demonstrating poor predictive values.8 Galactography in general demonstrates a higher sensitivity in finding intraductal abnormalities but suffers from poor specificity due to the nonspecific nature of “filling defects.”9 Other modalities currently under investigation include magnetic resonance enhanced galactography as well as molecular testing of nipple discharge cellular samples.10,11 Currently, there is no sufficient evidence that any diagnostic test or combination of preoperative tests can conclusively predict the lack of malignancy in patients with PND. This background provides the rationale for investigation of the use of modalities that visualize the ductal system in real time such as mammary ductoscopy to improve the diagnostic accuracy of duct excision for PND.
There are several studies that have evaluated the utility of mammary ductoscopy in the evaluation and treatment of patients with PND. To date, there have been no prospective, randomized clinical trials comparing the sensitivity and specificity of mammary ductoscopy to alternative modalities such as major duct excision or mammary galactography. However, in total the results of mammary ductoscopy in over 1000 patients with PND have been published, and these studies provide the best available evidence as to whether ductoscopy provides patient benefit.
Two endpoints that are frequently measured in the observational studies of ductoscopy are the diagnostic accuracy of the procedure in defining the source of the PND as well as the identification of clinically occult malignancy. Several large studies have demonstrated the utility of mammary ductoscopy in identifying intraductal pathology in patients with PND.5,12-14 Initial reports of mammary ductoscopy using a fiberoptic flexible scope demonstrated a fairly low proportion of patients (92 of 259 or 36%) where an intraductal papillary lesion was visualized.12 However, subsequent reports demonstrated visualization of intraductal abnormalities in over 90% of patients with PND.5,13 The increased identification of intraductal lesions was likely contributed by several factors including improvement of scope optics, better patient selection for single-duct defined nipple discharge, surgeon progression along the learning curve, and a loosening of the criteria for visualization of abnormalities with included categories such as “wall thickening” and intraductal “debris” as well as “stricture.” Dietz et al compared the ductoscopy findings with those identified during preoperative galactography and suggested a higher rate of visualization of intraductal pathology with ductoscopy as compared to galactography (90% vs 76%, respectively).5 In addition, in that particular study ductoscopy was able to identify additional papillary lesions downstream from the initial papilloma identified by galactography, which could have theoretically been left behind without a ductoscopy-directed excision. Other subsequent studies have demonstrated similar results, suggesting that the use of intraoperative ductoscopy might negate the need for preoperative galactography. Dooley et al reported the feasibility of office ductoscopy to determine which patients with pathologic nipple discharge should undergo duct excision.15 Conceptually, this approach merits further study, although the author does admit that poor sensitivity and specificity of ductal brushing and cytology and lack of intraductal biopsy is a limiting factor in terms of being able to reassure patients that they do not have malignancy.16
Although the use of ductal washing cytology is beyond the scope of this chapter, one noteworthy study that nicely correlated ductal cytology using a catheter-based system demonstrated a very low sensitivity (17%) for detection of malignant or atypical cells in patients with known cancer.17 However, as demonstrated by Sauter et al, the sensitivity and specificity of ductoscopy in combination with ductal washing cytology through the ductoscopy and image analysis could increase the sensitivity to identify malignancy to 92% with 60% specificity.18 The contrast between these studies as well as findings from other studies suggest that the cytologic yield from ductoscopy washings may be superior to that of nipple aspiration or ductal lavage catheters, and thus ductoscopy may prove to be a useful tool for obtaining representative cytology samples from deep within the ductal system for research purposes.
As mentioned previously, most studies to date have demonstrated that preoperative assessment of patients with PND using nipple discharge cytology, hemoccult testing, or routine mammography fail to predict the presence of malignancy in a significant proportion of patients.19,20Table 58-1 demonstrates cumulative results of several published series of large case series with respect to the identification rate of occult malignancy. The indications for performing duct excision as well as the method of ductoscopy varies among the studies cited and may explain the wide variance in identification of occult malignancy rates from 2% to over 24%, but overall the mean of over 600 patients is approximately 5%. Interestingly, several case series of the identification of occult malignancy in patients with PND undergoing duct excision without mammary ductoscopy also cite detection rates ranging from 9% to 13%.19,21,22 This brings into question whether the use of mammary ductoscopy increases the detection of occult malignancies beyond that identified by routine duct excision. Sharma et al performed a comparative analysis of 95 patients who underwent mammary ductoscopy during ductal excision to 140 patients who underwent routine major duct excision.23 Although the study was not randomized, the 2 groups were comparable in terms of factors that could increase the risk of the presence of breast cancer such as atypical cytology on preoperative nipple aspirate cytology, presence of abnormality on mammogram or preoperative galactography, and percentage of patients with a final diagnosis of atypical ductal hyperplasia. In reviewing the final pathology, the percentage of patients who had cancer visualized by ductoscopy was lower than that of patients who had a major duct excision (3% vs 9%). The volume of tissue removed in the ductoscopy group was also less than that removed during routine duct excision, and the authors concluded that the higher identification rate of occult malignancy could have simply reflected a better tissue sampling when ductoscopy was not used.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







