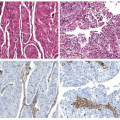
under UES. Overall, rearrangements of chromosome 6p21, 7p15, and 17q21 are frequent in ESS.7,8 By cytogenetic, polymerase chain reaction, and/or fluorescence in situ hybridization (FISH) analysis (Fig. 9-1), approximately half of ESSs carry a characteristic t(7;17)(p15;q21), resulting in gene fusion between JAZF1 and SUZ12.10 The same t(7;17) and gene fusion is also present in all endometrial stromal nodules examined to date.10,11 Normal unrearranged SUZ12 allele is also expressed in endometrial stromal nodules not expressed in ESS with t(7;17).12 Additionally, a small subset of cases lacking t(7;17) show 6p21 rearrangement, most often in the form of der(7)t(6;7)(p21;p22)5,10 with the rearrangement of 6p21 leading to the fusion of PHF1 with partner genes such as JAZF1 or EPC1.5,13 One case of ESS with t(X;17) involving rearrangement of SUZ12 was recently described.9 SUZ12 and PHF1 are both members of Polycomb group proteins, which participate in transcriptional silencing through chromatin remodeling, a mechanism that is crucial in cellular development.14 In ESS, the gene fusion likely results in the formation of functionally aberrant Polycomb complexes. Furthermore, although both endometrial stromal nodules and ESS carry t(7;17),
only ESS exhibits concurrent silencing of the unrearranged SUZ12 allele and would result in the complete abrogation of normal SUZ12 protein function. Although the precise oncogenic mechanism has not been elucidated, it would appear that abnormal transcriptional repression is central to oncogenesis of ESS. Even though abnormal expressions of Polycomb genes are commonly seen in other malignancies,14 genetic fusion/rearrangement of these Polycomb group members as demonstrated by cytogenetic and/or FISH analysis using, for example, the JAZF1 break-apart probe set (Fig. 9-1) has only been described for endometrial stromal nodules, ESS, and a subset of UES (suspected dedifferentiated ESS) to date. Therefore, this series of genetic aberrations represents a highly specific molecular diagnostic feature for the family of endometrial stromal tumors, even in cases with smooth muscle differentiation.2,11,15,16 However, the sensitivity of such molecular testing is inadequate because 40% to 50% of ESSs can lack these changes. There is currently also no diagnostic immunomarker that can be used to reliably distinguish ESS from other uterine sarcomas like ULMS. Further characterization of the full molecular/genetic spectrum of this disease perhaps using a next-generation sequencing approach is needed to improve the sensitivity of these diagnostic molecular tests.
Table 9-1 Classification of Uterine Sarcomas | |||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree