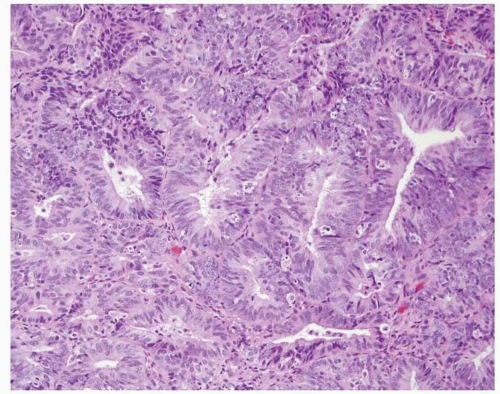
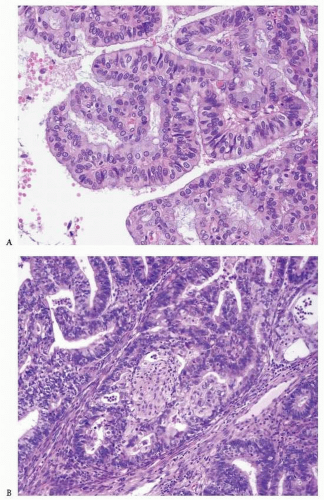
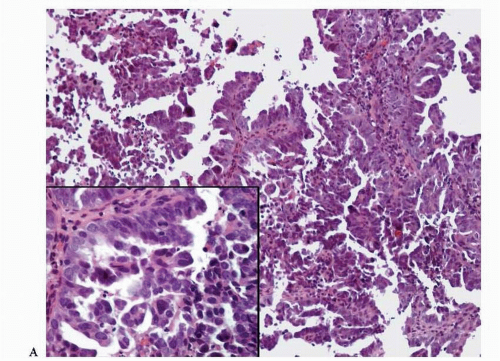
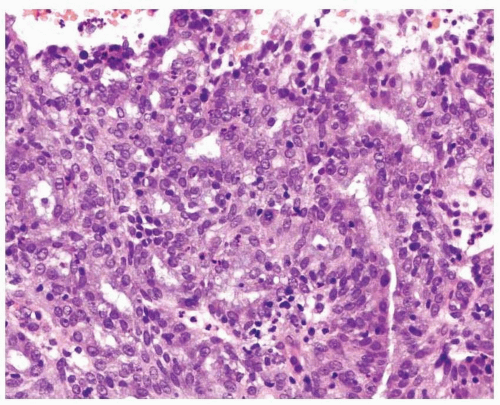
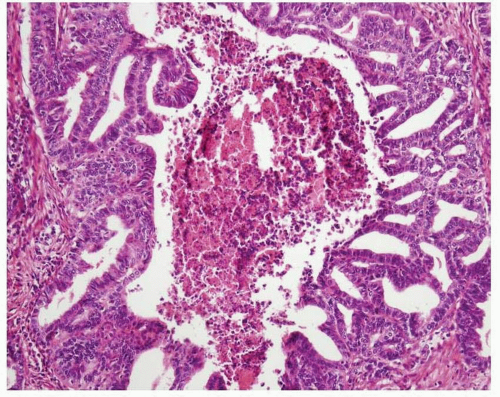
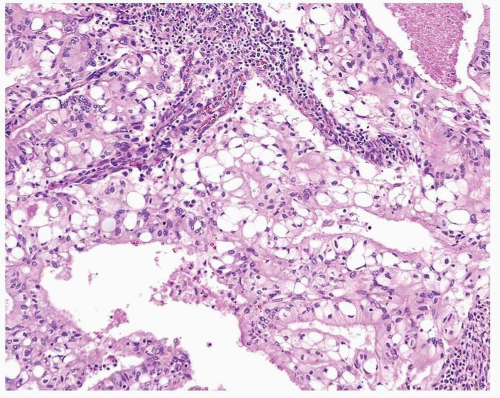
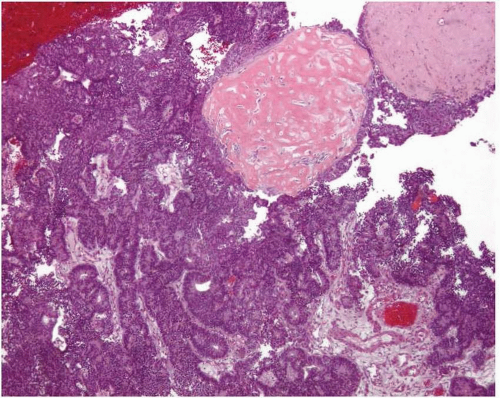
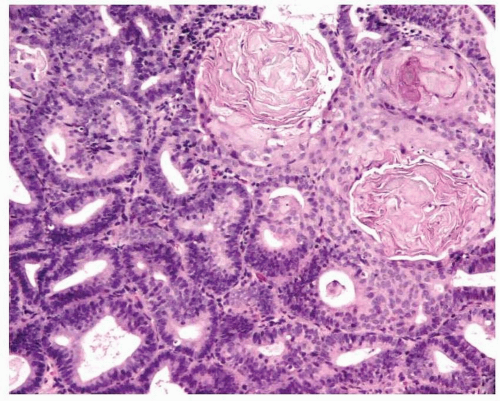
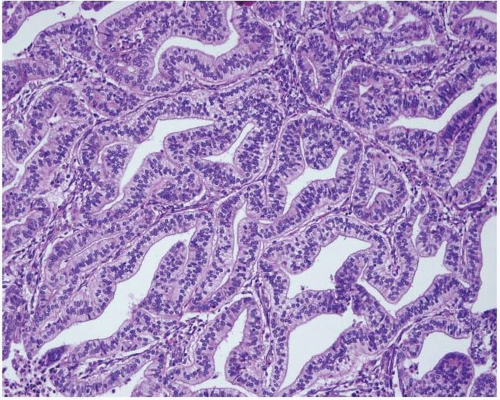
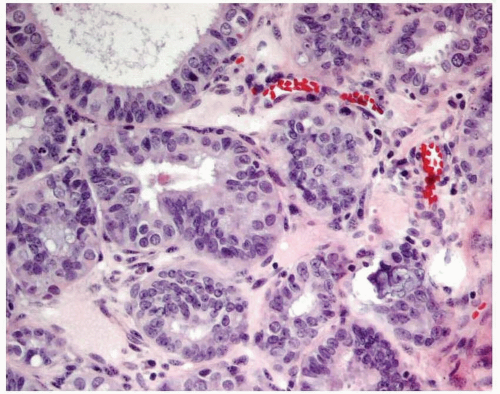
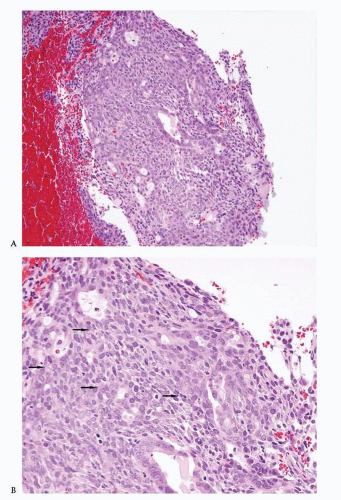
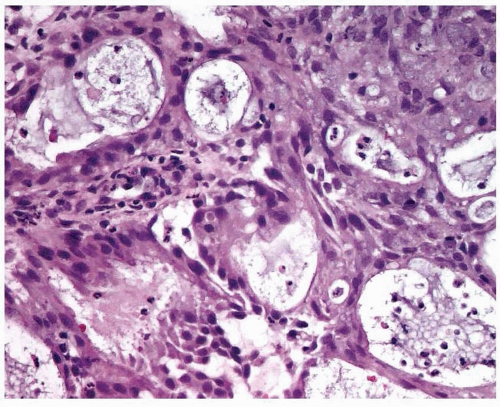
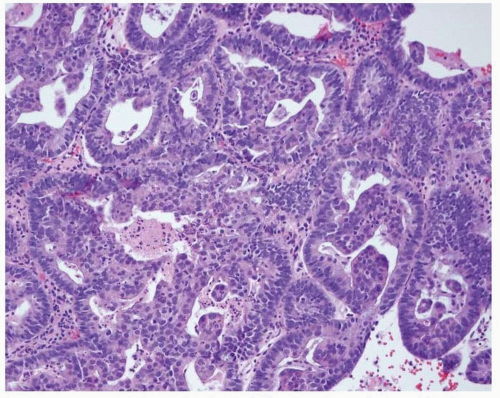
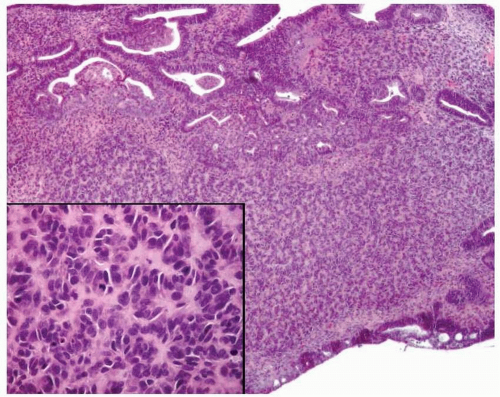
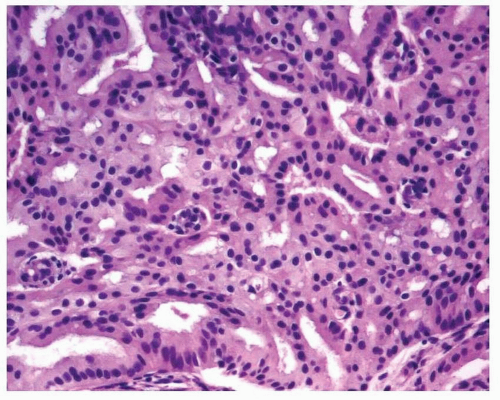
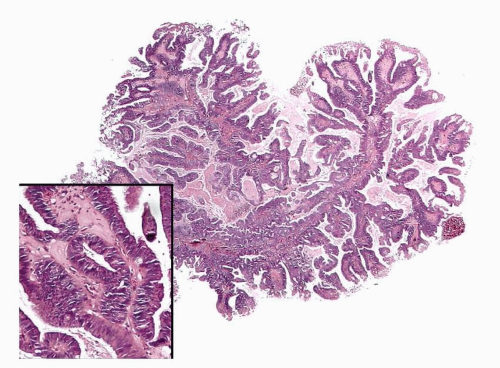
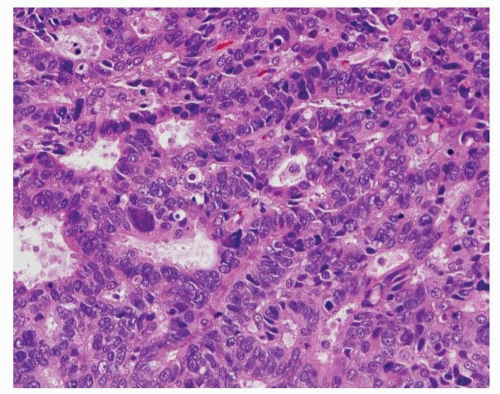
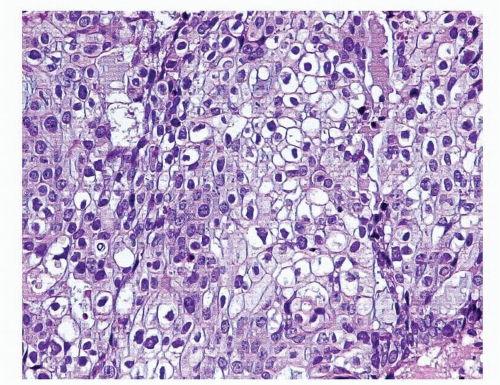
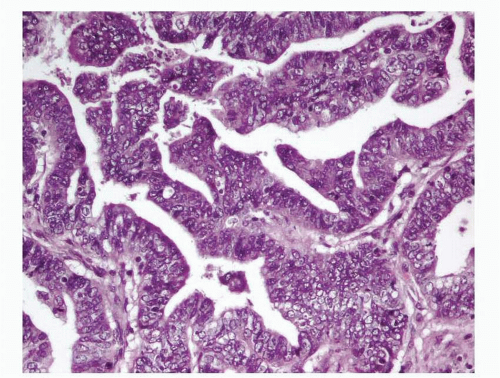
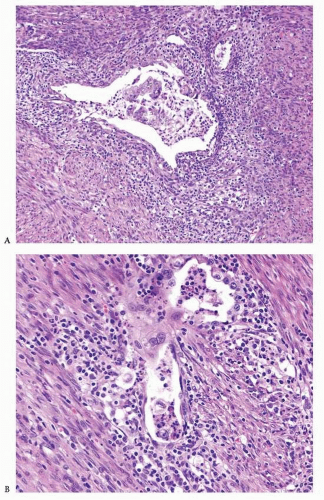
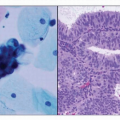
Endometrioid adenocarcinoma is the most common type of endometrial carcinoma, accounting for 80% of cases.
3 This tumor is composed of glands that resemble those seen in the normal endometrium. The variably sized glands seen in this type of tumor are usually oval or round, although they can be angulated or branched. They are lined by columnar cells with stratified or pseudostratified nuclei. The degree of nuclear atypia is usually mild to moderate. The cytoplasm is either basophilic, amphophilic, or slightly eosinophilic (
Fig. 4-1). Intraluminal mucin can be seen, and in some cases, this becomes a prominent finding. Intracellular mucin, usually focal and less frequently diffuse, may also be noted (
Fig. 4-2).
6,7 Ciliated cells, necrosis of the glands or within luminal spaces (
Fig. 4-3), foamy stromal cells, signet ring cells (
Fig. 4-4), and infrequently, intestinal differentiation, including goblet cells and argentaffin cells, can be seen.
7,8,9,10 and
11 In addition, psammoma bodies and focal, bland-looking heterologous elements (fat, cartilage, osteoid) are occasionally found (
Fig. 4-5).
12,13
Differential Diagnosis
Endometrioid Adenocarcinoma versus Complex Endometrial Hyperplasia with or without Atypia
One of the three following criteria is required for the diagnosis of endometrioid adenocarcinoma:
Back-to-back endometrial glandular proliferation occupying an area of at least 2 × 2 mm (
Fig. 4-15)
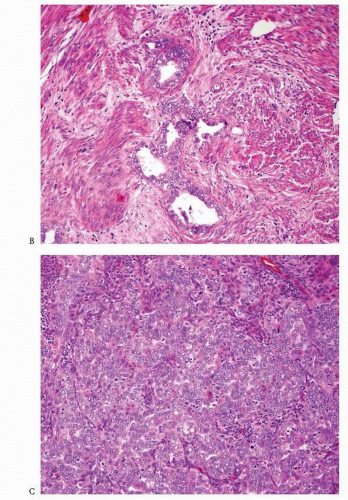
A desmoplastic or fibroblastic stroma infiltrated by irregular glands (
Fig. 4-17)
The last criterion mentioned earlier must be used with caution because the stromal reaction secondary to a recent endometrial curettage and the fibroblastic stroma of an endometrial polyp with torsion may mimic this feature.
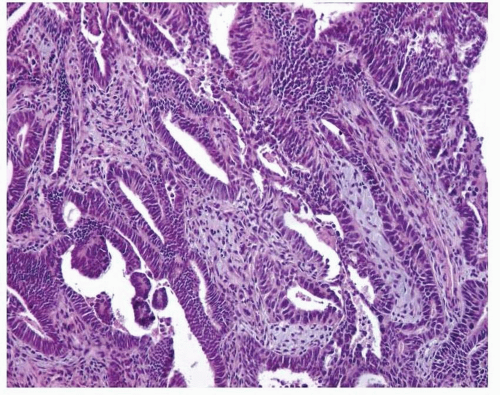
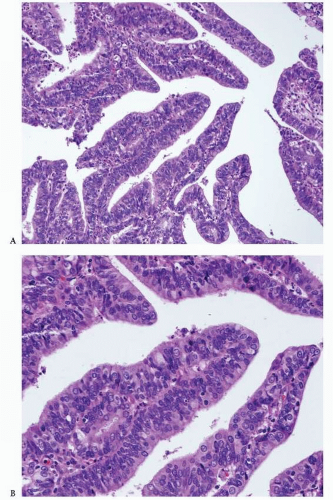
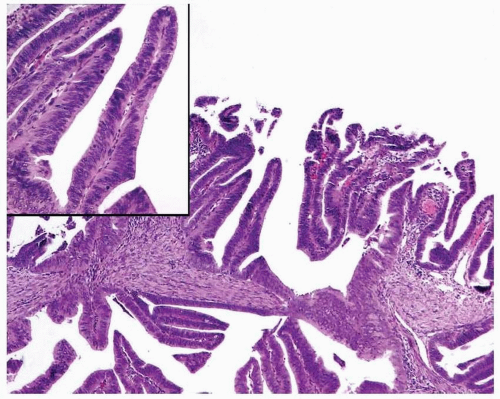
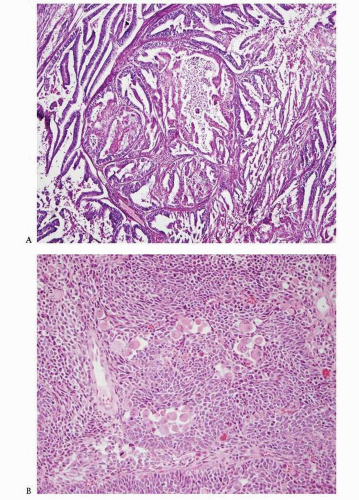
Endometrioid Adenocarcinoma with a Villoglandular Pattern versus Papillary Endometrial Adenocarcinoma of Intermediate Grade versus Serous Carcinoma
The papillae seen in the villoglandular pattern are slender and finger-like and lined by neoplastic cells with low-grade cytologic features and nuclei that preserve their polarity. In addition, the outer surface of the papillae is smooth. In contrast, the papillae seen in papillary endometrial adenocarcinoma of intermediate grade are long or short and lined by cells with moderate cytologic atypia and nuclei that have partially lost their polarity. The outer surface of the papillae is somehow irregular (
Figs. 4-18A-B and
4-19A-B). Serous carcinoma can have either long or blunt papillae, with or without fibroconnective cores, lined by markedly atypical cells usually showing conspicuous mitotic activity. Immunohistochemically, villoglandular adenocarcinoma and intermediate-grade papillary endometrial adenocarcinoma are usually either negative or focally positive for p53 (author’s unpublished observations), whereas serous carcinoma is usually positive for this immunomarker.
33,34
Endometrioid Adenocarcinoma versus Glandular Variant of Serous Carcinoma
The presence of marked cytologic atypia in an endometrioid-looking tumor composed mostly of glands should raise the diagnostic possibility of serous carcinoma, glandular variant (
Fig. 4-20). The use of an immunohistochemical panel that includes p53, progesterone receptor (PR), and
PTEN will assist in making the correct diagnosis. The combination of lack of p53 expression, positive PR expression, and loss of
PTEN is in keeping with the diagnosis of endometrioid adenocarcinoma.
34
Endometrioid Adenocarcinoma with Glycogenated Squamous Differentiation versus Clear Cell Carcinoma
Attention to histologic features is of utmost importance in making the correct diagnosis. In cases of endometrioid adenocarcinoma with glycogenated squamous differentiation (
Fig. 4-21), there are areas with overt squamous differentiation in the vicinity. In contrast, clear cell carcinoma usually demonstrates a combination of architectural patterns: solid, tubuloglandular, and papillary.
Endometrioid Adenocarcinoma with Sarcomatoid (Spindle Cell) Features or with Sex Cord-Like Pattern/Hyalinization versus Malignant Mixed Müllerian Tumor
Careful attention to histologic features will allow the correct diagnosis of these neoplasms. As mentioned earlier, endometrioid adenocarcinoma with sarcomatoid (spindle cell) features demonstrates areas where the spindle cell component merges with the glandular component, whereas endometrioid adenocarcinoma with sex cord-like pattern and hyalinization shows a constellation of typical features such as the formation of cords and clusters of spindle and epithelioid cells in a background of hyalinized material usually associated with squamous differentiation. The value of immunohistochemical studies, such as EMA and keratin, to enhance the epithelial nature of the spindle cells is limited because they can be negative or only focally positive in these neoplasms.
29 In addition, the sarcoma component of a malignant mixed müllerian tumor can be focally or diffusely positive for keratin and EMA.
35
Endometrioid Adenocarcinoma Associated with Primitive Neuroectodermal Tumor versus Malignant Mixed Müllerian Tumor
Rare cases of endometrioid carcinoma associated with primitive neuroectodermal tumor (PNET) have been described (
Fig. 4-22A-D). The neuroectodermal component is characterized by the presence of uniform oval, round, or slightly elongated cells with a scanty or moderate amount of cytoplasm arranged in sheets, nests, or trabeculae. The presence of perivascular rosettes, Homer Wright rosettes, ependymal-type rosettes, astrocyte-like cells in a fibrillary background, or ganglion-like cells is variable. Immunohistochemical studies show that cells with neuroectodermal differentiation are positive for neurofilament, synaptophysin, and CD99 (although some cases show nonspecific staining for the latter). These cells also are either negative or focally positive for keratin.
36,37 When molecular studies detect the rearrangement of the
EWSR1 gene, the diagnosis of peripheral PNET can be made. In contrast, cases lacking this finding should be designated as tumors with neuroectodermal differentiation or, alternatively, as central-type PNET rather than “PNET, not otherwise specified” to avoid confusion with the former. Of interest, some cases of uterine PNET are part of a malignant mixed müllerian tumor.
37 Combined endometrioid adenocarcinoma and PNET have been described in patients ranging from 47 to 71 years of age and have been associated with advanced-stage disease and a poor prognosis.
36,37
Endometrioid Adenocarcinoma, FIGO Grade 1, with Myometrial Invasion versus Atypical Polypoid Adenomyoma
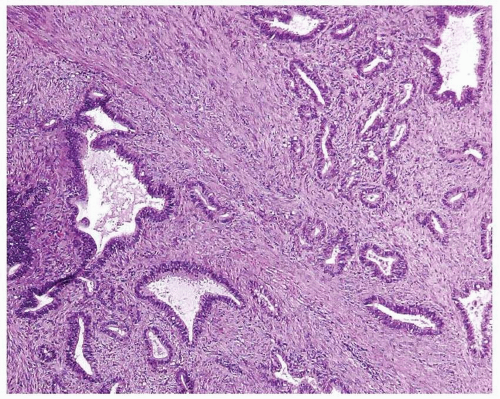
The diagnosis of atypical polypoid adenomyoma (APA) should be considered whenever a proliferation of endometrial glands is seen within a smooth muscle or fibromuscular background arranged in intersecting fascicles (
Fig. 4-23). In the typical case of APA, the glandular proliferation is similar to that seen in hyperplasia and tends to have squamous morules. The glandular size and shape are variable, as is the presence of cytologic atypia.
38 The following features distinguish APA from myoinvasive endometrioid adenocarcinoma, FIGO grade 1:
Young age (average age, 39 years)
The presence of intersecting fascicles of smooth muscle or fibro muscular tissue
A more cellular smooth muscle than the normal myometrium seen in cases of myoinvasive endometrioid adenocarcinoma
The presence of endometrioid proliferation exclusively in fragments containing smooth muscle or fibromuscular tissue
Endometrioid Adenocarcinoma Arising in the Endometrium versus Endometrioid Adenocarcinoma Arising in the Uterine Cervix
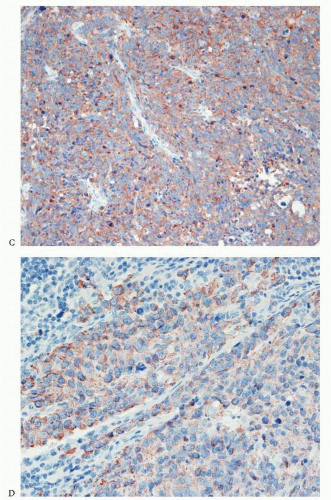
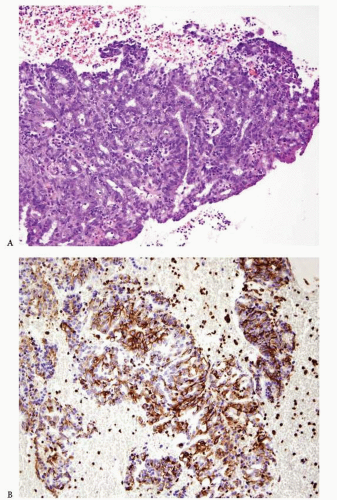
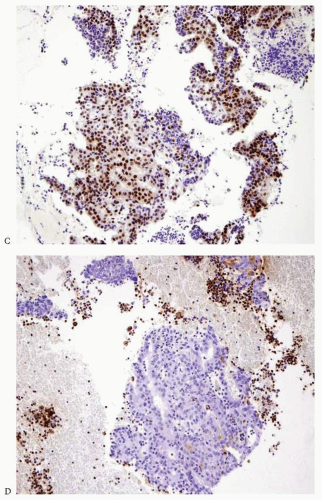
Carcinomas arising in the endometrium or endocervix may have overlapping histologic features. In cases where, upon review of the hematoxylin and eosin-stained slides, the origin of the tumor (i.e., endometrium versus endocervix) cannot be determined, the use of immunohistochemical studies is indicated. Tumors positive for vimentin and estrogen receptor (ER) and negative for CEA are favored to have an endometrial origin (
Fig. 4-24A-D), whereas tumors that are positive for CEA and negative for vimentin and ER are favored to be endocervical in origin.
39 p16 is another immunomarker recommended to assist in the distinction between these two types of tumor. It is usually diffusely expressed by adenocarcinomas of endocervical origin and has patchy expression in endometrioid endometrial adenocarcinomas
40; however, there are two caveats to bear in mind: (1) p16 is also diffusely expressed in high-grade endometrial adenocarcinomas (serous, clear cell, and grade 3 endometrioid), and (2) in a small biopsy, a tumor with patchy expression of p16 can falsely appear to have diffuse expression of this marker. Human papillomavirus (HPV) in situ hybridization is another tool used to confirm the cervical origin of a given tumor; however, up to 30% of cases of cervical carcinoma can be negative for this test.
41 In a small subset of cases, the results of these ancillary tests are discordant, and imaging studies and/or fractioned curettage are required to determine with certainty the site of origin of the tumor.