Endometrial Cancer
 ANATOMY
ANATOMY
The uterus is a hollow, muscular organ located in the true pelvis between the bladder and the rectum. The average adult uterus is about 8 cm long, 5 cm wide, and 2.5 cm thick. It is divided into the fundus, body (corpus), and cervix. The junction between the body and cervix is called the isthmus. The fundus is pierced at each cornu by the fallopian tubes. The uterine surface is partially covered by peritoneum. The uterine cavity is lined by endometrium, made up of columnar cells forming many tubular glands. The thickness of the endometrium varies during the menstrual cycle, but by the end of menstruation it should be 2 to 3 mm in thickness. The wall of the uterus is composed of myometrium, consisting of smooth muscle fibers. The major supports of the uterus are the broad, round, uterosacral and cardinal ligaments. The major blood supply to the uterus is the uterine artery, which enters the uterus at the isthmus after it crosses over the ureter. The lymphatic drainage for the body of the uterus is mainly to the obturator and internal and external iliac lymph nodes. The lymphatics from the fundus accompany the ovarian artery and drain into the para-aortic lymph nodes.
 EPIDEMIOLOGY AND RISK FACTORS
EPIDEMIOLOGY AND RISK FACTORS
Endometrial cancer is the most common gynecologic cancer and the fourth most frequently diagnosed cancer in women in the United States. According to 2011 cancer statistics, the estimated number of newly diagnosed cases is 46,470, with a probability of 1 of every 39 women (2.58%) developing it during her lifetime.1 Although it is a cancer that affects predominantly postmenopausal women, 5% to 30% of women are <50 years of age at the time of diagnosis.2,3 The expected number of deaths from endometrial cancer in 2011 was 8,120, making it the eighth-leading cause of death from cancer in women. Prior estimates on the death rate from endometrial cancer seemed to indicate that the rate was on the rise, but the most recent data show that the death rate of 4.18 per 100,000 women did not change from the year 1990 to 2007.1
The age-standardized rate per 100,000 for endometrial cancer in more developed areas of the world is 12.9, with a cumulative risk of 1.6% (age 0 to 74 years), compared to 5.9 and 0.7%, respectively, in less developed areas, indicating a possible influence of environmental factors on the incidence of this disease.4 The exact etiology of endometrial cancer remains unknown, but several risk factors have been associated with it, chiefly unopposed estrogen. It is well established that endometrial cancer risk is increased among women who have high circulating levels of bioavailable estrogens and low levels of progesterone, so that the mitogenic effect of estrogens is insufficiently counterbalanced by the opposing effect of progesterone.5–7 The source of unopposed estrogen could be endogenous or exogenous. In a case–control study, the association between endogenous estrogen and endometrial cancer was demonstrated. In that study there was correlation between high blood concentrations of estrogens and increased risk of endometrial cancer.7 Lifetime cumulative number of menstrual cycles, that is, menstruation span, is associated with increased risk of developing endometrial cancer. This is due to the fact that endometrial cell proliferation increases during the follicular phase, which is the longest in the menstrual cycle. Thus, early age at menarche (estimated relative risk [RR], 1.5 to 2) and late age at menopause (RR, 2 to 3), examples of increased menstruation span, are risk factors for endometrial cancer.8,9 Nulliparity (RR, 3) is also associated with increased risk of endometrial cancer8 due in part to anovulatory menstrual cycles. Obesity increases endometrial cancer risk (RR, 5) mainly through changes in endogenous hormone metabolism. After menopause, when ovarian production of both estrogen and progesterone ceases, the major source of estrogen is via peripheral conversion, mostly within adipose tissue, of androgens that continue being produced by the adrenal glands and ovaries. Thus with obesity there is an increase in the amount of bioavailable estrogens in the circulation and the endometrial tissue.7,10,11 Obesity may also influence endometrial cancer risk via chronic hyperinsulinemia, which appears to be a key factor for the development of ovarian hyperandrogenism, associated with anovulation and progesterone deficiency, especially for premenopausal women.10 Non–insulin-dependent diabetes mellitus and hypertension (RR, 1–3) also increases the risk of endometrial cancer. This is often believed to be secondary to obesity, but there are data showing that these risk factors could be independent of obesity.12,13,14 With regard to exogenous estrogen, it is well established that the use of estrogen-only hormone-replacement therapy and sequential oral contraceptives greatly increases endometrial cancer risk (RR, 10 to 20), whereas combined preparations, that is, those that contain a progestogen as well as estrogen throughout the treatment period, have a protective effect (RR, 0.3 to 0.5).15–16,17 The use of tamoxifen in patients with breast cancer has been associated with increased risk (RR, 3 to 7) of endometrial cancer.18,19–20 The mechanism of action of tamoxifen is in competition with that of endogenous estrogen for estrogen receptors. In premenopausal women, tamoxifen has an antiestrogenic effect, but in postmenopausal women it has a weak estrogenic effect because of the upregulation of estrogen receptors. In a recent meta-analysis on adjuvant tamoxifen and endometrial cancer, for patients who were <55 years of age there was little absolute risk compared to patients in of 55 to 69 years of age, for whom the 15-year incidence was 3.8% in the tamoxifen group versus 1.1% in the control group (absolute increase 2.6% [standard error 0.6], 95% confidence interval [CI] = 1.4 to 3.8), highlighting the influence of age on the risk of endometrial cancer from tamoxifen use.21 Initial data seemed to indicate that the majority of endometrial cancers associated with tamoxifen use were of early stage with favorable features.22 More recent data, however, show a change in the profile of these endometrial cancers, with a rise in the rate of serous, clear-cell, carcinosarcoma, and sarcoma types.23,24 Inherited genetic predisposition, especially in the setting of hereditary nonpolyposis colorectal cancer (HNPCC), probably accounts for <5% of all endometrial cancer cases. Mutations in one of the four mismatch repair genes hMLH1, hMSH2, hMSH6, or hPMS2 have been identified in patients with Lynch syndrome. Although HNPCC is thought of primarily in terms of risk of developing colorectal cancer, it is important to note that lifetime cumulative risk of endometrial cancer for women with HNPCC is 40% to 60%, which equals or exceeds their risk of colorectal cancer.25 There seems to be a high rate of lower uterine segment involvement in patients with HNPCC-associated endometrial cancer.26
 CLINICAL PRESENTATION AND NATURAL HISTORY
CLINICAL PRESENTATION AND NATURAL HISTORY
The most common presentation for endometrial cancer is postmenopausal vaginal bleeding, which is reported by 80% to 90% of patients. The incidence of endometrial cancer in women presenting with postmenopausal bleeding is only 10% to 15%. This incidence, however, could range from 1% up to 25%, depending on patient age and the presence of other risk factors. In a recent repot of a total of 3,548 women presenting with postmenopausal vaginal bleeding, 201 (6%) had a diagnosis of endometrial carcinoma. Use of a multiple logistic regression model showed that recurrent episodes of bleeding (odds ratio [OR], 3.64), a history of diabetes (OR, 1.48), older age (1.06), and high body-mass index (OR, 1.07) increased the risk of endometrial malignancy when corrected for other characteristics.27 Other patterns of presentations include vaginal discharge, abnormal Papanicolaou smear, or thickened endometrium on routine transvaginal ultrasound. For patients with advanced disease, they may present with urinary or rectal bleeding, constipation, pain, lower-extremity lymphedema, abdominal distension due to ascites, and cough and/or or hemoptysis.
The International Federation of Gynecology and Obstetrics (FIGO) annual report28 showed that the 5-year survival rate for 8,110 patients with endometrial cancer treated between 1999 and 2001 was 80%. Such excellent outcome is a reflection of the fact that the majority of patients are diagnosed with early-stage disease. The tumor was limited to the corpus uteri in 71% of cases, involved the cervix in 12%, and extended beyond the uterus, but short of distant spread, in 13%. For patients with disease limited to the endometrium or with <50% myometrial invasion, the 5-year survival rate was 91%. However, the rate dropped to 66% when disease extended to adnexa/serosa/positive peritoneal cytology, to 57% with regional lymph node involvement, to 25.5% with bladder or rectal involvement, and to 20% with distant spread. For clinically staged patients, the 5-year survival rate ranged from 67% for early-stage disease down to 15% for advanced disease. Mass screening for endometrial cancer in women at average risk or increased risk due to a history of unopposed estrogen therapy, tamoxifen therapy, late menopause, nulliparity, infertility or failure to ovulate, obesity, diabetes, or hypertension is not recommended. American Cancer Society (ACS) recommends that women at average and increased risk should be informed about risks and symptoms (in particular, unexpected bleeding and spotting) of endometrial cancer at the onset of menopause and should be strongly encouraged to immediately report these symptoms to their physician. However, screening has been recommended by the ACS for women who carry, or are related to carriers of, the HNPCC mutation, starting at age 35 years, including annual transvaginal ultrasound and endometrial biopsy.29 Prophylactic hysterectomy and bilateral salpingo-oophorectomy once childbearing is completed have been shown to effectively reduce the risk of endometrial cancer in patients with HNPCC and should be strongly considered.30
FIGURE 70.1. Sagittal view of the uterus on transvaginal ultrasound. A: Normal thin endometrium (arrow). B: Thickened endometrium (arrow).

 DIAGNOSTIC WORKUP
DIAGNOSTIC WORKUP
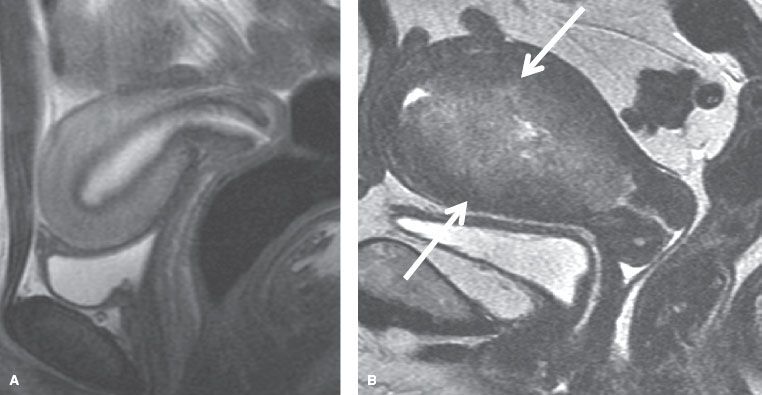
Endometrial tissue sampling remains the gold standard by which the diagnosis of endometrial cancer is established. This is achieved via biopsy or dilatation and curettage (D&C). Endometrial biopsy, which can be easily performed in the office with a Pipelle or similar device, is the preferred approach. Its sensitivity in detecting endometrial cancer in postmenopausal women is 99.6% compared to 91% in premenopausal women. Its specificity is >98% for both groups.31 If the patient is undergoing hysterectomy, routine D&C is not necessary after an office Pipelle sampling has documented malignancy. However, if symptoms persist, the office sampling is inadequate, or the patient is being considered for conservative fertility-sparing approaches, a D&C should be performed. In addition, D&C provides more reliable assessments of final pathologic findings in hysterectomy specimens, mainly with regard to tumor grade.32 Given that the incidence of endometrial cancer in women with postmenopausal bleeding is only 10% to 15%, it is unclear how feasible it is to perform endometrial sampling on every patient. Transvaginal ultrasonography (TVU) may be considered as a useful tool to assess patient’s vaginal bleeding.33 Normal endometrium looks thin and homogeneously hyperechoic, but it is thickened and heterogeneous, with hyperplasia, polyps, and cancer,34 as shown in Figure 70.1. The consensus statement from the Society of Radiologists in Ultrasound defines an endometrial thickness of 5 mm or greater as being abnormal.35 If the thickness of the endometrium is <5 mm, the risk of endometrial cancer is minimal; the false-negative rate is about 4%. Under such circumstances, endometrial sampling may be foregone if no further episodes of vaginal bleeding occur.33 Recent meta-analysis seems to indicate that perhaps a cut-off of 3-mm thickness rather than <5 mm provides even better diagnostic accuracy.36 If the TVU is abnormal but the biopsy is negative/nondiagnostic or the uterine cavity is inaccessible, then saline-infusion sonography or hysteroscopy should be considered to help exclude intracavitary lesions, especially polyps that might contain cancer.37,38 In addition, these methods are also helpful in premenopausal women, for whom the accuracy of TVU is limited because the endometrial thickness fluctuates, depending on the level of female hormones. The potential downside to saline infusion or hysteroscopy is that there have been reports that the insufflation of the distending medium into the canal has been associated with an increase in positive peritoneal cytology, although the prognostic implications are unclear of such positive cytology “induced” by sampling.39 Several imaging studies are available to define the extent of disease preoperatively. Good-quality pelvic computed tomography (CT) scans obtained with oral and intravenous contrast can demonstrate the extent of the endometrial tumor. The endometrial carcinoma is a hypodense mass relative to the normal myometrium and may be seen as a diffuse, circumscribed vegetative or polypoidal mass within the uterine cavity. If myometrial invasion is seen, it usually implies involvement of greater than one-third to one-half of the myometrial thickness. Involvement of the cervix is seen on CT as cervical enlargement >3.5 cm in diameter with heterogeneous low-attenuation areas within the fibromuscular stroma. Parametrial or sidewall extension is seen by the loss of periureteral fat in the former and <3 mm of intervening fat between the soft tissue mass and the pelvic sidewall in the latter. Involvement of the fallopian tubes and ovaries is detected in the usual fashion, and for lymph nodes is >1 cm in diameter in the short axis.40,41 Magnetic resonance imaging (MRI) is considered the most accurate imaging study to assess tumor extension in endometrial cancer, especially myometrial invasion. Dynamic contrast-enhanced MRI is the optimal MRI method for detecting myometrial invasion,42 with an accuracy of 85% to 93%. A clear junctional zone or preservation of a sharp delineation between the tumor and the myometrium implies disease limited to the endometrium. Disease characterized by disruption of the junctional zone, increased–signal-intensity tumor in the inner half of the myometrium with preservation of the outer myometrium, or both correlate with superficial myometrial invasion. If there is extension of the high–signal-intensity tumor into the outer myometrium with preservation of a peripheral rim of normal, intact myometrium, then that is considered deep myometrial invasion (Fig. 70.2). MRI also helps to delineate tumor extension into the cervix. The normal cervical stroma is hypointense on T2-weighted images and is replaced by intermediate–signal-intensity tumor in cases of invasion.34 The reported sensitivity of MRI in detecting lymph node metastasis is 27% to 66% and the specificity is 73% to 94% in surgically staged patients.43 Positron emission tomography/computed tomography (PET/CT) is also being used in endometrial cancer. There seems to be little benefit in assessing the primary tumor extension. With regard to regional lymph node metastasis, the reported sensitivity is 50% to 100%, the specificity is 87% to 100%, and the accuracy is 78% to 100%. The main limitation of PET/CT is its inability to detect metastasis in lymph nodes ≤5 mm in size.43 The FIGO staging for endometrial cancer is a surgical staging, and thus preoperative imaging studies (except chest x-rays) are not part of the staging. Cancer antigen 125 (CA 125) serum levels could be elevated in patients with endometrial cancer. Kim et al.,44 in a review of 413 patients, found that 23.9% of patients had >35 U/mL serum CA 125 levels. Hsieh et al.45 found that preoperative levels of >40 U/mL correlated significantly with regional lymph nodes metastasis and suggested that such levels could be used as an indication for full pelvic and periaortic lymphadenectomy at the time of surgical staging in the absence of metastatic disease.
FIGURE 70.2. Sagittal magnetic resonance imaging view of the uterus. A: Normal uterus. B: Deep myometrial invasion (arrows).
Pathologic Classification
Endometrial Hyperplasia
The diagnostic criterion for hyperplasia is an increase in the number and size of proliferating glands. The International Society of Gynecologic Pathologists standardized the subclassification of endometrial hyperplasia. In simple hyperplasia, there is only glandular proliferation and enlargement with increased stromal cellularity. This rarely progresses to carcinoma (<1%). Complex hyperplasia is characterized by back-to-back proliferation of glands with intraluminal papillae, epithelial pseudostratification, and few mitotic figures. If there is no cytologic atypia, the risk of malignant degeneration is again quite low, on the order of 3%. Any proliferation demonstrating cytologic abnormalities (in cellular or nuclear morphology) is classified as atypical hyperplasia. Atypical hyperplasia has a much higher risk of progression to an invasive carcinoma—8% for simple atypical hyperplasia, increasing to 29% for complex hyperplasia associated with atypia.46 The GOG conducted a prospective trial in which all patients with atypical hyperplasia of the uterus underwent an immediate hysterectomy. The rate of underlying concurrent carcinoma in the uterus was 42.6% in these patients.47 The standard recommended treatment for atypical hyperplasia of the uterus is hysterectomy if childbearing is complete and the patient has no other contraindications to surgery. In patients who desire future fertility or have an absolute contraindication to surgery, progestational therapies may be used with caution.48
TABLE 70.1 PATHOLOGIC CLASSIFICATON OF ENDOMETRIAL CANCERS

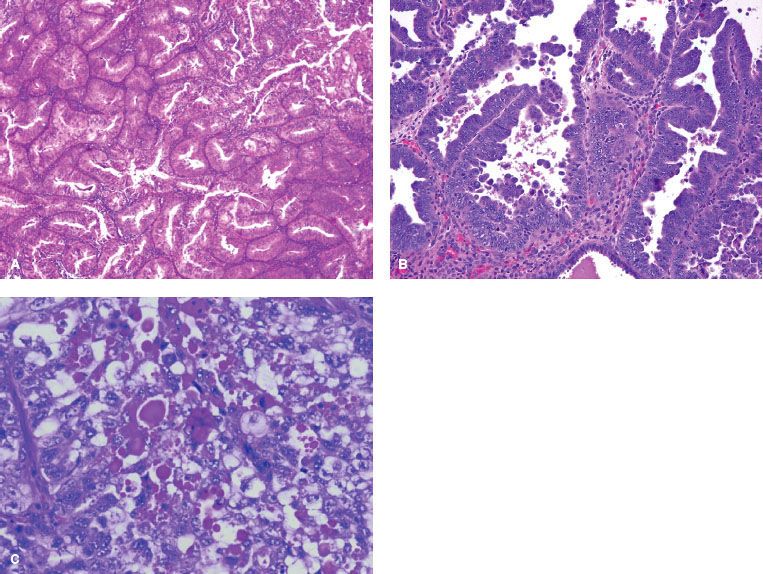
FIGURE 70.3. Different histologic types of endometrial cancer. A: Endometrioid. B: Papillary serous. C: Clear cell.
Carcinoma of the Endometrium
Endometrioid Carcinoma
Endometrioid adenocarcinoma is the most common endometrial carcinoma, constituting 75% to 80% of all cases (Table 70.1). The classic histologic appearance is that of marked glandular proliferation with back-to-back proliferation of glands and little intervening stroma (Fig. 70.3A). The name endometrioid is derived from resemblance to proliferative-phase endometrium. Architectural grading is determined by the amount of solid mass of tumor cells compared to well-defined glands. Grade 1 is an endometrioid cancer in which <5% of the tumor growth is in solid sheets. Grade 2 is an adenocarcinoma in which 6% to 50% of the tumor is composed of solid sheets of cells. Grade 3 occurs when >50% of the tumor is made up of solid sheets. Nuclear grading is determined by the nuclear shape, size, chromatin distribution, and size of the nucleoli. The grading is primarily driven by the architectural grading, but if there is marked nuclear atypia in an otherwise grade 2 architectural grading, it should be increased to grade 3. Within endometrioid adenocarcinoma, the subtypes are endometrioid carcinoma not otherwise specified (NOS), endometrioid carcinoma with squamous differentiation, villoglandular endometrioid carcinoma, secretory carcinoma, and a ciliated cell variant.49 Most of the endometrioid adenocarcinomas are designated NOS. Foci of squamous differentiation are often found with endometrioid adenocarcinoma. The squamous component could be benign, with the designation of adenoacanthoma, or malignant, in which case it is called adenosquamous carcinoma. Such designations have not been very useful, however, because the degree of differentiation of the squamous component parallels that of the glandular architectural grading. Therefore, most gynecologic pathologists use the term adenocarcinoma with squamous differentiation. Other subtypes of endometrioid adenocarcinoma include the relatively common villoglandular carcinoma, which grows in a papillary fashion. The prognosis of this subtype is similar to that of low-grade endometrioid cancer, and it must not be confused with serous carcinoma because of its papillary features. Secretory carcinoma, which represents <2% of all endometrial carcinomas, is characterized by a very well differentiated glandular pattern with much intracellular glycogen, thus resembling early secretory endometrium. Although the cells have clear cytoplasm, their histologic and cytologic features are different from those of clear-cell carcinoma. Ciliated carcinoma is a very rare subtype, characterized by the presence of ciliated cells comprising >75% of the tumor specimen. It is usually associated with a history of prior estrogen use, and the prognosis is quite good, since most are well differentiated.
Mucinous Carcinoma
This designation requires >50% of the tumor cells to be mucinous. These cells are carcinoembryonic antigen positive and are laden with mucin, which stains positively with mucicarmine and periodic acid–Schiff stains but is diastase resistant. Because of the resemblance to endocervical adenocarcinoma, it is essential to exclude it by endocervical curettage. Mucinous carcinomas are usually well differentiated and have the same prognosis as ordinary endometrioid carcinomas.
Serous Carcinoma
Serous carcinomas, also known as papillary serous cancers, resemble ovary cancer in terms of histology and to some extent in terms of behavior. The mere presence of papillary structure is not diagnostic because other histologic types may have papilla as well. However, the presence of marked cellular atypia in addition to papilla distinguishes serous carcinoma from others (Fig. 70.3B). Psammoma bodies are found in up to 33% of cases. The incidence of serous endometrial cancer is about 10% that of endometrial carcinomas. This is a very aggressive subtype, with a high propensity for early lymphatic and intraperitoneal dissemination, often despite little myometrial penetration.50 In the FIGO annual report, the 5-year survival rate was 52.6% compared to 83.2% for endometrioid carcinoma.28
Clear-Cell Carcinoma
Clear-cell carcinoma of the endometrium resembles renal carcinoma, but its origin from Müllerian structures is now well established. Unlike vaginal and cervical clear-cell carcinoma, it is not related to intrauterine diethylstilbestrol exposure. The microscopic structure may vary from solid patterns to glandular differentiation (Fig. 70.3C). In the latter pattern, small cells resembling “hobnail” cells line spaces and glands. These are cells that extruded their cytoplasm, leaving bare nuclei that protrude into the glandular lumens. The prognosis of this cancer is somewhat similar to that of serous cancer. In the FIGO annual report, the 5-year survival rate was 62.5% compared to 83.2% for endometrioid carcinoma and 52.6% for serous carcinoma.28
Squamous Carcinoma
This type of cancer is extremely rare, and the diagnosis has to be made after the exclusion of cervical origin. The 5-year survival rate based on the FOGO report is 68.9% overall, but the prognosis is poor for patients with extrauterine disease or distant spread.28
Undifferentiated Carcinoma
The World Health Organization classification describes endometrial undifferentiated carcinomas as “malignant poorly differentiated endometrial carcinomas, lacking any evidence of differentiation” without any further characterization.51 Undifferentiated carcinomas can also be associated with an endometrioid carcinoma component, and such tumors have been referred to as “dedifferentiated carcinomas,” which is being recognized with increased frequency. Some of these tumors may belong to the spectrum of gynecologic neoplasms seen in the setting of microsatellite instability and possibly Lynch syndrome.52
Mixed Histology
Mixed-cell-type endometrial cancer composed of two or more pure types is not uncommon. By convention, in order to be designated as mixed, the other cell-type component has to comprise at least 10% of the tumor. Except for mixed endometrioid and serous or clear-cell carcinoma, the clinical significance of mixed cell type is questionable.
Simultaneous Tumors
Cancers of identical type may be discovered in the ovary and endometrium simultaneously. Usually, the site of the largest tumor is assigned the primary origin, but occasionally true primary endometrial and ovarian malignancies may coexist. This field effect of the Müllerian system may occur in as much as 15% to 20% of ovarian endometrioid tumors.53 If the endometrial tumor is <5 cm in diameter, well differentiated, with no vascular invasion, limited to less than the middle one-third of the myometrium, and the ovarian lesions are bilateral, it is more likely that there are two concomitant primary tumors. Genetic profiling may represent a powerful tool in clinical practice for distinguishing between metastatic and dual primaries in patients with simultaneous ovarian/endometrial cancer and for predicting disease outcome.54
Molecular Biology
Several investigators pointed out that there are two distinct types of endometrial cancer.55,56 In type I endometrial cancer there is strong correlation with prior estrogen stimulation. The cancers in this category are often indolent in nature, with minimal myometrial invasion and low-grade histology. They affect premenopausal and perimenopausal women. Type II endometrial cancer often affects postmenopausal women with no prior history of estrogen stimulation. The histology of the tumors is often high grade, such as serous or clear-cell cancers with deep invasion, and at a more advanced stage at the time of presentation. What is intriguing is the fact that at the molecular level, the existence of two distinct types of endometrial cancer seems to be validated. In a recent review by Dedes et al.,57 the compiled data from the literature show that the most frequently altered molecular pathway in type I endometrial carcinomas is the PI3 K/PTEN/AKT pathway, which is dysregulated by oncogenic mutations, PTEN loss of function, and/or overexpression of upstream tyrosine kinase growth factor receptors, leading to uncontrolled cell proliferation and survival. On the other hand, the main pathway alterations in type II endometrial cancers involve the tumor suppressors p53 and/or p16, which cause cell cycle dysregulation and genetic instability. Other features frequently observed in type II cancer are loss of E-cadherin expression and the amplification and overexpression of HER2. Inactivation of the p53 tumor suppressor gene is seen in almost 90% of cases of serous carcinoma.58,59 Mutation in the p53 gene, however, is encountered in only 10% of endometrioid adenocarcinoma, with most occurring in grade 3 tumors. Inactivation of the cell cycle regulator p16 is also more frequent in type II (40%) than in type I (10%). The underlying mechanism is not clear but probably involves deletion and promoter hypermethylation.60 Reduction in the levels of the adhesion molecule E-cadherin is more frequent in type II (62% to 87%) than in type I (5% to 53%) tumors.61,62 HER2 overexpression or amplification is seen in 17% to 32% of type II compared to 3% to 10% in type I tumors.63–64,65 In contrast, mutation in the PTEN tumor suppressor gene is found in 30% to 50% of type I endometrial cancer. PTEN mutations have been detected in endometrial hyperplasia with and without atypia (19% and 21%, respectively), which suggests that PTEN mutations are early events in the development of endometrial cancer.60 Mutations in PIK3CA occur in 36% of type I endometrial cancer and coexist frequently with PTEN mutations.60 Mutation in K-ras proto-oncogene is seen in 10% to 30% of endometrial cancer patients.59 Microsatellite instability (MSI), which is found in patients with HNPCC, is also seen in approximately 20% of “sporadic” endometrial cancers.66,67 MSI, mutations in PTEN/PIK3CA, and mutations in K-ras frequently coexist within the same tumor.68 B-Catenin is important for cell differentiation, maintenance of normal tissue architecture, and signal transduction. B-Catenin mutations are seen in 25% to 40% of type I endometrial cancer. Of interest, the mutations do not usually coexist with MSI and mutations in PTEN/PIK3CA and K-ras. This suggests that type I endometrial cancers with B-catenin mutations may develop via a unique pathway.68 Microarray analysis has further revealed distinct gene expression profiles among different histologic types of endometrial cancer.69,70
TABLE 70.2 ENDOMETRIAL CANCER SURGICAL STAGING SYSTEM: INTERNATIONAL FEDERATION OF GYNECOLOGY AND OBSTETRICS 1988

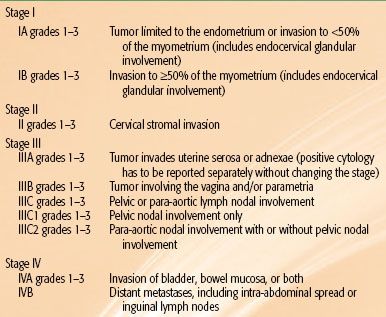
TABLE 70.3 REVISED ENDOMETRIAL CANCER SURGICAL STAGING SYSTEM: INTERNATIONAL FEDERATION OF GYNECOLOGY AND OBSTETRICS 2009
 STAGING
STAGING
Before 1988, the staging system for endometrial cancer was clinical. Stage I was tumor limited to the uterus, with IA designation if the length was ≤8 cm and IB if it was >8 cm. Stage II was for when cervix was involved, stage III when disease extension beyond uterus/cervix was limited to the true pelvis, and stage IV when it extended beyond the true pelvis or involved bladder or rectum (IVA) or distant spread (IVB). This system is applicable to the few patients who cannot have surgery and are treated with definitive radiation. Creasman et al.71 reported a Gynecologic Oncology Group (GOG) study on 621 patients with clinically stage I endometrial cancer, that is, confined to the corpus, who underwent total abdominal hysterectomy/bilateral salpingo-oophorectomy, peritoneal cytology, and selective pelvic and para-aortic lymphadenectomy. Of the 621 patients, 144 (22%) were found to have disease outside the uterus. The rate of positive peritoneal cytology was 12%, that of adnexal involvement was 5%, and that of regional lymph node involvement was 11%. Pelvic node metastases were found in <3% of patients with grade 1 endometrium-confined disease but in >30% when grade 3 disease penetrated the outer one-third of the myometrium. Aortic nodal disease, although rare in grade 1 disease or in the absence of pelvic node metastasis, was seen in 14% and 23% of patients with deeply invasive grade 2 or 3 disease, respectively. This highlighted some of the shortcomings of the clinical staging system and led to the adoption of a surgical staging system by FIGO in 1988 in order to better estimate 5-year prognoses for patients and to better tailor adjuvant therapy to those patients most likely to benefit from it (Table 70.2).
In 2009 the FIGO staging system was modified again.72 Patients who formerly were staged as IB, that is, <50% myometrial invasion, are now considered IA. Patients with >50% myometrial invasion are designated as stage IB. Endocervical glandular involvement no longer affects staging; only patients with cervical stromal invasion are considered stage II. Having positive peritoneal cytology no longer affects staging. Parametrial extension is now considered IIIB. Patients with stage IIIC are now subdivided into IIIC1 if pelvic nodes are involved and IIIC2 if para-aortic nodes are involved (Table 70.3). The discriminating power of the new FIGO staging system is being debated. Page et al.73 evaluated 10,839 cases from 1998 to 2006 using the Surveillance, Epidemiology, and End Results (SEER) Program. The analysis demonstrated the usefulness of two divisions rather than three for stage I in the new FIGO staging system. In contrast, a study from Memorial Sloan-Kettering Cancer Center (MSKCC) of 1,307 patients with FIGO 1988 stage I disease showed that the revised system for stage I did not improve its predictive ability over the 1988 system.74
Prognostic Factors
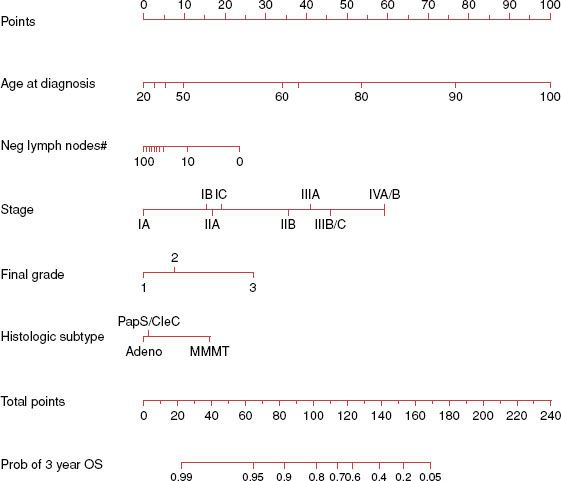
Several clinicopathologic factors have been identified in patients with endometrial carcinoma to help predict the prognosis and individualize the treatment plan. At MSKCC, a nomogram was developed for predicting overall survival of women with endometrial cancer (n = 1,735) after primary therapy.75 Use of five prognostic factors—age, grade, histologic type, number of lymph nodes removed, and FIGO 1988 surgical stage—predicted OS with high concordance probability (Fig. 70.4).
Age
The influence of older age on worse outcome has been well established. The adverse impact of older age is often explained by pointing out that older patients tend to present with aggressive histology and more-advanced disease and are generally treated less aggressively. What is intriguing, however, is that the strongest correlation between older age and poor outcome is seen in patients with favorable characteristics. Age ≥60 years has been shown to be predictive of local-regional recurrence (hazard ratio [HR], 3.9; p = .0017) and death (HR, 2.66; p = .01) in a randomized trial limited to stage I and in which patients with deep myometrial invasion grade 3 were excluded.76 The adverse impact of advanced age on outcome persists even when elderly patients are treated as aggressively as their younger counterparts.77
FIGURE 70.4. Nomogram for predicting overall survival in patients with endometrial cancer.
Race
White women tend to fare better than African Americans, independent of other prognostic factors.78 It is important to note that although the prevalence of endometrial cancer is lower in African American women, the incidence of high-risk tumors in this group is higher.79
Histologic Subtype
According to the FIGO annual report, the 5-year survival rate was 83.2% for endometrioid adenocarcinoma, compared to 52.6% for serous cancer and 62.5% for clear-cell cancer in 8,033 surgically staged patients. Patients with endometrioid histology have surgical stage III to IV disease only in 13.8% of patients, compared to 41.7% with serous and 33% with clear-cell carcinoma, which could explain the worse outcome. However, the influence of histology was seen even in patients with surgical stage I disease (n = 5,285), for whom 5-year survival dropped from 90% for endometrioid histology to 79.9% with serous and 85.1% with clear-cell carcinoma.28
Grade
Tumor grade is one of the most sensitive indicators of prognosis. Grade directly affects the depth of myometrial penetration and the frequency of lymph node involvement. Most grade 1 tumors are limited to the endometrium or have superficial myometrial penetration, and the overall risk of pelvic and para-aortic lymph nodal metastases is 3% and 1.5%, respectively. Only 10% of grade 1 tumors have deep myometrial invasion, and pelvic and para-aortic lymph nodal involvement in these is 12% and 6%, respectively. Conversely, >50% of grade 3 tumors have >50% myometrial invasion, and these have pelvic and para-aortic nodal involvement on the order of 30% and 20%, respectively.71 In the FIGO annual report,28 grade 3 was an independent predictor of poor survival on multivariate analysis within each stage—stage I (HR, 2.45), II (HR, 2.14), III (HR, 2.44), and IV (HR, 2.55).
Myometrial Invasion
Regardless of grade, only 1% of tumors limited to the endometrium had lymph nodal involvement, as compared with 25% pelvic and 17% para-aortic involvement with deep penetration.71 Before the 1988 FIGO staging system, the depth of invasion had been reported as none or inner, middle, or outer one-third of the myometrium. The 1988 FIGO staging system subdivided myometrial invasion into none or inner or outer half. Under that staging system, for patients with <50% myometrial invasion, it seems that invasion to less than versus greater than one-third is not a significant predictor of outcome.80 In the current 2009 FIGO staging system, depth of invasion in stage I is divided into two categories: A (no or <50% myometrial invasion) and B (>50% invasion).
Lymphovascular Invasion
This is seen in about 15% of the cases of endometrial cancer. The GOG study found that lymphovascular invasion (LVI)–positive tumors were associated with a 27%, or fourfold, increase in the pelvic lymph nodal metastases, and a 19%, or sixfold, increase in para-aortic nodal metastases.71 This translates into more frequent relapses, including vaginal recurrences,81 and a poorer outcome.82
Lower Uterine Segment Involvement
The GOG study of surgical-pathologic spread patterns found a doubling of the incidence of pelvic nodal involvement from 8% to 16% and an increase in para-aortic nodal involvement from 4% to 14% when the tumor arose from or involved the isthmus.71 There seems to be a high rate of lower uterine segment involvement in patients with HNPCC-associated endometrial cancer.26
Cervical Involvement
In the 1988 FIGO staging system, cervical involvement was divided into IIA when limited to endocervical glandular involvement and IIB when it involves the cervical stroma. According to the FIGO report, the 5-year survival for stage IIA was very good (89.9% for grade 1 and 83.7% for grade 2). In contrast, the corresponding figures for stage IIB were 81.2% and 76.9%, respectively.28 This led to a change in the 2009 FIGO staging system, in which only cervical stromal invasion is considered stage II. Although the prognosis of the old stage IIA grades 1 and 2 approximated stage I rather than stage IIB, it is important to note that in the same FIGO annual report, patients with stage IIA grade 3 did not fare as well; their 5-year survival was 68.3%, which was worse than that for IC grade 3 (74.9%) and similar to that for IIB grade 3 (64.9%).
Peritoneal Cytology
Peritoneal fluid positive for malignant cells is found in 12% to 15% of all patients undergoing surgical staging. This is associated with 25% pelvic lymph node involvement and 19% para-aortic node involvement.71 The data suggest a higher rate of positive cytology for patients undergoing laparoscopic-vaginal hysterectomy, in which there is manipulation of the uterine cavity, compared to total abdominal hysterectomy, in which there is no such manipulation.84 The literature regarding the true impact of positive peritoneal cytology is mixed. One confounding factor, mainly in patients with no other risk factors, is whether all endometrial cancer cells that gained access to the peritoneal cavity are capable of independent growth. In a review of the literature, Wethington et al.85 found that the overall incidence of positive washings is approximately 11%. Patients with grade 1 or 2 disease, no evidence of cervical involvement, <50% myometrial invasion, and no LVI were considered low risk. In patients with positive peritoneal cytology, the rate of recurrence for low-risk patients was 4.1% compared to 32% for those considered high risk (p < .001). This indicates the association of malignant cytology with other adverse prognostic factors. In the recent FIGO staging (2009), having positive peritoneal cytology is no longer considered stage IIIA.
Adnexal/Serosal Involvement
About 5% of patients with stage I and occult stage II disease have adnexal involvement.71 This is associated with a fourfold increase in lymph node metastases; thus, pelvic lymph nodal positivity rises to 32% (as compared with 8% without adnexal spread), and para-aortic nodal involvement is seen in 20% (as opposed to only 5% in patients with no adnexal spread). The incidence of serosal involvement is less common. Jobsen et al.86 reported on 46 patients with isolated adnexal involvement and 21 with isolated serosal involvement. There was no statistically significant difference in outcome between adnexal and serosal involvement. The 5-year disease-free survival was 76.4% versus 59.6% (p = ns), and the disease-specific survival was 76.3 % and 75.4%, respectively.
Pelvic and Para-Aortic Lymph Node Involvement
The pattern of lymphatic spread in endometrial cancer is different than that in cervical cancer. In endometrial cancer, a simultaneous spread to both pelvic and para-aortic nodes could occur, whereas in cervical cancer the spread to para-aortic nodes is almost always secondary to pelvic lymph node involvement. Overall, about 11% of patients with stage I and occult stage II endometrial cancer have pelvic nodal involvement. This increases to 25%, 30%, and 50% with deep myometrial invasion, adnexal involvement, and extrauterine spread, respectively.71 Lymph node involvement is a major predictor of outcome; the 5-year disease-free survival rates drop to 65% to 70% in patients with pelvic lymph node involvement as their only risk factor.87 The rate of para-aortic nodal metastases is about 5% of all patients with stage I and occult stage II disease. The biggest risk factor for para-aortic node involvement is the presence of pelvic nodal metastases; more than 30% of patients with pelvic nodal involvement have para-aortic disease. The 5-year disease-free survival rates drop to about 30% in this subpopulation.87
Molecular Prognostic Factors
The application of molecular biology tools to endometrial cancer has provided insights into the pathogenesis of the disease and may lead to early detection, as well as to development of novel therapeutic strategies.88 Mutations of the tumor suppressor gene p53 have been most extensively studied. There is a consistent observation linking the overexpression of p53 with advanced stage and poorer outcome.89,90 Overexpression of HER-2 is also associated with more advanced disease and poor outcome.65 PTEN mutation is associated with early-stage, nonmetastatic disease and more favorable survival outlook.91 Data in the literature suggest a favorable survival outlook associated with microsatellite instability in endometrioid endometrial cancers.92 As our knowledge regarding the molecular biology of endometrial cancer matures, risk stratification may soon be based on molecular alterations rather than pathologic variables.
 SURGICAL MANAGEMENT
SURGICAL MANAGEMENT
Surgery is the main treatment for endometrial cancer. It consists of simple hysterectomy, bilateral salpingo-oophorectomy (BSO), and inspection of the pelvic and abdominal cavities, with biopsy of any suspicious extrauterine lesions, accompanied in most cases by peritoneal washings. Surgical assessment of lymph nodes ranges from palpation, biopsy of suspicious nodes, to pelvic and para-aortic lymphadenectomy.
Hysterectomy
There are several approaches to simple hysterectomy, also known as extrafascial hysterectomy, but in the main it consists of removal of the entire uterine corpus and cervix without contiguous parametrial tissue. The pubocervical fascia is entered, and the ureters are not unroofed. Total abdominal hysterectomy/BSO (TAH/BSO) is the most prevalent and time-tested form of simple hysterectomy in endometrial cancer. It is an abdominal approach, usually via a vertical midline incision that allows thorough exploration of intra-abdominal and pelvic cavities. The main drawback of TAH/BSO is, that it is a laparotomy-based approach, in a group of patients with pre-existing comorbidities such as obesity, hypertension, and diabetes. Therefore, it is not surprising that minimally invasive surgery, whether laparoscopically or robotically, has gained a great deal of acceptance in the surgical management of endometrial cancer. In laparoscopic vaginal hysterectomy/BSO (LAVH/BSO) the uterus is removed vaginally rather than abdominally. The benefit of using the laparoscope is to enable the surgeon to have a thorough intra-abdominal exploration and to perform BSO, which is difficult to accomplish with just a vaginal hysterectomy. The GOG completed a trial in which patients with clinical stage I to occult IIA uterine cancer were randomly assigned to laparoscopy (n = 1,696) or open laparotomy (n = 920), including hysterectomy, salpingo-oophorectomy, pelvic cytology, and pelvic and para-aortic lymphadenectomy. The main study endpoints were 6-week morbidity and mortality, hospital length of stay, conversion from laparoscopy to laparotomy, recurrence-free survival, site of recurrence, and patient-reported quality-of-life outcomes.93 Laparoscopy had fewer moderate to severe postoperative adverse events than laparotomy (14% vs. 21%, respectively; p < .0001). Hospitalization of >2 days was significantly lower in laparoscopy than in laparotomy patients (52% vs. 94%, respectively; p < .0001). The conversion rate to laparotomy was 25.8%. With a median follow-up time of 59 months for 2,181 patients still alive, there were 309 recurrences (laparoscopy, 210, laparotomy, 99) and 350 deaths (laparoscopy, 229; laparotomy, 121). The estimated 5-year recurrence rate was 11.61% in the laparotomy arm and 13.68% for laparoscopy. The estimated 5-year overall survival rate was 89.8% for laparoscopy and 89.8% for laparotomy. The study demonstrated that surgical treatment of endometrial cancer can be performed laparoscopically with relatively small differences in recurrence rates (estimated difference at 3 years, 1.14%). These results, combined with improved quality of life and decreased complications associated with laparoscopy, are reassuring to patients and allow surgeons to reasonably suggest this method as a means to surgically treat and stage patients with presumed early-stage uterine cancers.94 In recent years, robotic–assisted hysterectomy/BSO has emerged as an alternative minimally invasive surgery in endometrial cancer. It affords many advantages, including three-dimensional visualization, increased freedom of instrument movement, and enhanced ergonomics and surgeon comfort. The question of difference in cost is under debate debatable.95 Radical hysterectomy is not routinely performed in endometrial cancer due to low incidence of parametrial involvement. There is no evidence to show that the cure rates are any better with such radical operations. The possible exception to this might be in patients with gross cervical involvement.96
FIGURE 70.5. Sentinel lymph node. Solid arrow points to the blue dye in an external iliac node. Dashed arrow points to a lymphatic channel draining to the sentinel node.
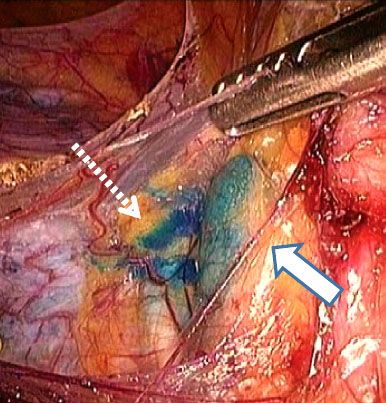
Lymphadenectomy
The question of which patients need routine surgical lymph nodal staging and, if so, to what extent is a matter of great debate. The uncertainty about lymphadenectomy relates to whether the benefit from it is prognostic rather than therapeutic. Those who advocate for no lymphadenectomy and limit nodal assessment to inspection and removal of any enlarged/suspicious pelvic or para-aortic nodes cite the lack of documented survival advantages to lymphadenectomy. Furthermore, patients who have adverse pathologic features that increase the risk of microscopic lymph node metastasis are generally offered adjuvant pelvic radiation. Advocates for full pelvic and para-aortic lymph node sampling reason that surgical staging is the most accurate method to assess the extent of disease and that the sensitivity and specificity of palpation of lymph nodes are only 72% and 81%, respectively.97 Lymphadenectomy in endometrial cancer includes removal of the fat pads surrounding the major vessels in the abdomen and pelvis without skeletonizing them. According to the GOG surgical guidelines, pelvic lymph nodes are to be removed from the distal one-half of the common iliac artery down to the circumflex iliac vein, and nodal tissue is to be removed anterior to the obturator nerve and surrounding the iliac arteries and vein. The para-aortic nodes include those overlying the vena cava, between the vena cava and aorta, and to the left of the aorta. The cephalad boundary of the para-aortic specimen is generally, but not limited to, the inferior mesenteric artery, and the distal boundary is the midpoint of the common iliac artery.93 For the sampling to be adequate, five lymphatic stations need to be removed—para-aortic, common iliac, internal iliac, external iliac, and obturator—or total of 10 nodes. Optional lymphadenectomy is another approach, in which preoperative tumor grading with intraoperative assessment of depth of myometrial invasion, as well as histologic subtype, is frequently used to decide whether lymph node dissection is necessary at the time of hysterectomy. With such a policy, patients with grade 3 disease or serous or clear-cell histology and those with deep myometrial invasion on frozen section will undergo lymphadenectomy. Opponents of selective lymphadenectomy point out that depth of invasion on frozen section correlated with final pathology in only 67% of cases.98 With regard to grade, preoperative FIGO grade 1 diagnosis correlates with final grade diagnosis in only 85% of cases of endometrial cancer.32
Despite the misgivings about optional or no lymphadenectomy, many surgeons have not embraced full lymphadenectomy. In a study of 27,063 women with unstaged endometrioid uterine cancer, lymphadenectomy was performed in only 30% of patients.99 Two trials addressed the role of lymphadenectomy. The first was an Italian study100 in which 514 eligible patients with preoperative FIGO stage I endometrial carcinoma were randomly assigned to undergo pelvic lymphadenectomy (n = 264) or no lymphadenectomy (n = 250). The median number of lymph nodes removed was 30 in the pelvic lymphadenectomy arm. Both early and late postoperative complications occurred more frequently in patients who had received pelvic systematic lymphadenectomy (81 patients in the lymphadenectomy arm and 34 patients in the no-lymphadenectomy arm; p = .001). Lymphadenectomy improved surgical staging, as statistically significantly more patients with lymph node metastases were found in the lymphadenectomy arm than in the no-lymphadenectomy arm (13.3% vs. 3.2%; p < .001). At a median follow-up of 49 months, the 5-year disease-free and overall survival rates in an intention-to-treat analysis were similar between arms (81.0% and 85.9% in the lymphadenectomy arm and 81.7% and 90.0% in the no-lymphadenectomy arm, respectively). In the second trial (Medical Research Council [MRC]/A Study in the Treatment of Endometrial Cancer [ASTEC]) patients with endometrial cancer believed preoperatively to be confined to the uterine corpus were first randomized to standard surgery consisting of hysterectomy-BSO, pelvic washing, and palpation of para-aortic nodes (n = 704) or to lymphadenectomy (n = 704). In the lymphadenectomy group patients underwent standard surgery plus systematic dissection of iliac and obturator nodes.101 If the nodes could not be dissected, sampling of suspect nodes was recommended. Whether to dissect the para-aortic nodes was left to the discretion of the surgeon. After a median follow-up of 37 months, 191 women (88 standard surgery group, 103 lymphadenectomy group) had died, with an absolute difference in 5-year overall survival of 1% (95% CI = 4 to 6) and an absolute difference in 5-year recurrence-free survival of 6%. The conclusion from both trials was that pelvic lymphadenectomy significantly improved surgical staging, that is, it is a good prognosticator, but it did not improve disease-free or overall survival. As a trade-off between lymphadenectomy and no surgical assessment at all in patients with endometrial cancer, there has interest in adopting a sentinel lymph node approach similar to that in breast cancer (Fig. 70.5). In a recent report from MSKCC, 266 patients with endometrial cancer underwent sentinel lymph node (SLN) mapping. At least one sentinel node was identified in 223 (84%) cases. Location of SLN was in the pelvis in 94% of cases, in the pelvis and para-aortic in 5%, and in the para-aortic in 1%. Positive nodes were diagnosed in 32 of 266 (12%) patients.102 In a prospective trial from France, at least one SLN was detected in 111 of the 125 eligible patients. Of the 111 patients, 19 (17%) had pelvic-lymph-node metastases. Three patients had false-negative results (two had metastatic nodes in the contralateral pelvic area and one in the para-aortic area), giving a negative predictive value of 97% (95% CI = 91 to 99) and sensitivity of 84% (95% CI 62 to 95). SLN biopsy up-staged 10% of patients with low-risk and 15% of those with intermediate-risk endometrial cancer.103 The results from these two studies suggest that SLN mapping is feasible and that adding SLN mapping to surgical staging procedures seems to increase the likelihood of detecting metastatic cancer cells in regional lymph nodes. Whether sentinel lymph node mapping will replace lymphadenectomy needs to be determined.
 ROLE OF RADIATION
ROLE OF RADIATION
Radiation therapy plays a significant role in the management of endometrial cancer. It is often used as an adjuvant treatment after surgery (which will be discussed here) or as definitive treatment for patients who are medically inoperable or with local recurrence (which will be discussed later). In the past, most patients were treated with preoperative intracavitary brachytherapy with or without external-beam radiotherapy, followed by hysterectomy. This approach is not without its merit, especially in patients with gross cervical involvement. However, most patients nowadays undergo surgery first; then, depending on the prognostic features obtained from the pathology review, the need for radiotherapy is determined. In recent years there has been a plethora of data from prospective, randomized trials addressing several aspects of the management of endometrial cancer. However, unlike in cervical cancer, for which the data from the majority of the randomized trials pointed in the same direction, that is, chemoradiation is better than radiotherapy (RT) alone, the data in endometrial cancer are less conclusive. Therefore, it is important for radiation oncologists to be familiar with the methodology of these studies so that objections to the use of any form of adjuvant RT can be addressed with facts.
Role of RT in Stages I and II
Treatment options for patients with early-stage endometrial cancer after hysterectomy include observation, intravaginal RT, or pelvic RT. At MSKCC, intravaginal RT is the preferred approach for most patients because it provides the best therapeutic ratio. As the discussion will demonstrate, observation may have the best morbidity profile, but it does not provide the best therapeutic ratio because of the increased risk of local recurrence. Pelvic RT, on the other hand, although very effective in reducing recurrence, has a higher morbidity profile than intravaginal RT. The results of prospective, randomized trials will be discussed first, and then treatment recommendations based on risk factors will follow.
Results of RT Randomized Trials
There are six randomized trials regarding the role of adjuvant RT in early-stage endometrial cancer, mainly pertaining to endometrioid histology and conducted in the “modern” era.
Observation Versus Pelvic RT
Three randomized trials compared pelvic RT to observation in early-stage endometrial cancer. The first trial was the Postoperative Radiation Therapy in Endometrial Cancer (PORTEC) trial, which randomized 715 patients after total abdominal hysterectomy and bilateral salpingo-oophorectomy (TAH/BSO) to observation or pelvic RT.104 Patients included were those with stage (FIGO 1988) IB grades 2 and 3 and those with IC grades 1 and 2. Those with IB grade 1 and those with IC grade 3 were excluded because it was felt that adjuvant RT was not indicated for the former and most physicians would not omit it for the latter. No lymph node sampling was done, and the dose of pelvic RT was 46 Gy at 2 Gy per fraction. At 5 years there was a statistically significant difference in the rates of vaginal/pelvic recurrence in favor of adjuvant pelvic RT (14% vs. 4%; p < .001). Overall survival, however, was not different between the two groups (81% RT vs. 85% surgery; p = .31), and the complications with pelvic RT were significantly higher (25% vs. 6%; p < .0001). In addition, many of the patients who relapsed locally after surgery alone were successfully salvaged with subsequent definitive RT. The second randomized trial was GOG 99. There were 190 patients with stage (FIGO 1988) IB to IIB (grades 1 to 3), who all underwent TAH/BSO, pelvic washing, and pelvic/para-aortic lymph node sampling and then were randomized to observation versus pelvic RT to a dose of 50.4 Gy at 1.8 Gy per fraction.105 At 2 years there was a statistically significant difference in the rates of relapse in favor of the adjuvant pelvic RT arm (3% vs. 12%; p = .007). The 2-year estimated incidence of isolated vaginal/pelvic recurrence was 1.6% in the RT group and 7.4% in the surgery-alone group. There was, however, no significant difference in 4-year overall survival (92% RT vs. 86% with surgery alone; p = .557), but there were more complications with pelvic RT. The third trial consisted of two trials with separate randomizations consolidated into one intergroup trial. One trial was conducted by the MRC and the other by the National Cancer Institute of Canada (NCIC). Furthermore, the MRC ASTEC trial in itself consisted of two trials with separate randomizations designed to answer a surgical as well as a radiation question.106 The surgical question was discussed earlier and regarded the need for lymphadenectomy in clinical localized endometrial cancer.101 The radiation question was whether pelvic RT is needed. Intermediate- or high-risk early-stage patients were then randomized to observation or pelvic RT. Intermediate risk included stage (FIGO 1988) IA grade 3, IB grade 3, IC grades 1 and 2, and IIA grades 1 and 2. High risk included IC grade 3, IIA grade 3, and IA to IIA serous and clear-cell tumors. Patients who had positive pelvic nodes (stage IIIC) were allowed but not those with cervical stromal invasion (IIB). The pelvic RT was given to a total dose of 40 to 46 Gy in 20 to 25 fractions over 4- to 5 weeks. Intravaginal RT was permitted regardless of the pelvic RT randomization as long as it was the stated policy for the treating center to do so. The recommended dose was 4 Gy in two fractions prescribed to a depth of 0.5 cm treating the upper one-third of the vagina when using high dose rate and 15 Gy when using low dose rate. The NCIC had a similar design but with a few exceptions. Patients with stage IIA serous or clear-cell carcinoma were excluded, as were those with positive nodes. The dose of pelvic RT was 45 Gy in 25 fractions, and intravaginal brachytherapy was permitted in accordance with local practice.
Of the 905 patients (789 MRC and 116 NCIC) in the trial, 453 were randomized to observation and 452 to pelvic RT. The two arms were balanced except for more high-risk patients (113; 25%) in the observation arm than in the pelvic RT arm (89; 20%). There were 137 (32%) patients in the observation arm in whom nodes were removed, and 5 (4%) had positive nodes. In the pelvic RT arm, 159 (38%) had nodes removed, and 6 (4%) were positive. In the observation arm, 228 (51%) patients received intravaginal RT, 7 (2%) received pelvic and intravaginal RT, 3 (1%) received pelvic RT, and 3 (1%) were unknown. Only 212 (47%) received no form of adjuvant RT. Conversely, in the pelvic RT arm, 24 (5%) did not receive any adjuvant RT, 10 (2%) received intravaginal RT, and 2 (0.4%) were unknown. Combined intravaginal and pelvic RT was given to 232 (52%) patients, and only 184 (41%) received pelvic RT alone. The primary endpoint of this study was overall survival. With a median follow-up of 58 months, the 5-year overall survival was 84% in both arms (p = .77). The 5-year cumulative incidence for isolated vaginal or pelvic recurrence was 6.1% in the observation group and 3.2% in the pelvic RT group. This difference was statistically significant (p = .2) with a HR of 0.46 (95% CI = 0.24 to 0.89). The rate of any acute toxicity was 27% in the observation arm compared to 57% for pelvic RT. Similarly, late toxicity was more prevalent in the pelvic RT compared to observation (61% vs. 45%, respectively).
The triad of lack of overall survival advantage, increased toxicity, and high salvage rate of local recurrence for patients who are observed have led many to conclude that all forms of adjuvant RT, not simply pelvic RT, should be abandoned. The morbidity of pelvic RT and the validity of omitting adjuvant RT in favor of RT for salvage policy will discussed later in the chapter. With regard to overall survival it is considered the gold standard for primary endpoint in many randomized trials in oncology, but for early-stage endometrial cancer it is perhaps unattainable with adjuvant RT for two reasons. First, many of the patients have other, competing causes of death such as hypertension, diabetes, and obesity. In the PORTEC trial the 8-year actuarial rates of intercurrent death were 19.7% in the RT arm and 15.6% in the surgery-alone arm.107 Endometrial cancer–related deaths in comparison were only 9.6% and 7.5%, respectively. Similarly, in the GOG 99 trial,105 approximately half of the deaths were due to causes other than endometrial cancer or treatment (surgery alone, 19 of 36; RT, 15 of 30). This led the authors of GOG 99 to write, “With this number of intercurrent deaths in both arms, even if RT reduces the risk of endometrial cancer-related deaths, the size of this trial is not adequate to reliably detect an overall survival difference.” That is why overall survival was not the primary endpoint in GOG99 but rather the disease-free interval, which was significantly different in favor of adjuvant pelvic RT over surgery alone.105 Therefore, it is not unreasonable to conclude that neither PORTEC nor GOG 99 was large enough to conclusively show whether adjuvant pelvic RT affected overall survival. The second difficult hurdle for adjuvant RT to overcome when discussing overall survival has to do with its localized nature. In the MRC/NCIC trial, the rate of first vaginal/pelvic recurrence was reduced from 11.4% (n = 37 of 453) in the observation arm to 2.8% (n = 13 of 452) with pelvic RT. Adjuvant pelvic RT, however, did not affect distant spread; the rate of first distant spread was 8.1% (n = 37 of 453) in the observation compared to 9% (n = 41 of 452) in the pelvic RT arm.106 One would expect adjuvant RT to make a difference in overall survival only when systemic therapy makes has an effect on the rate of distant spread. This is exactly the story learned from postoperative chest wall irradiation in breast cancer. Because pelvic RT significantly improved local control, albeit with increased toxicity, why not replace it with intravaginal RT rather than advocating observation for all early-stage endometrial cancer?
Observation Versus Intravaginal RT
In a trial reported by Sorbe et al.,108 645 patients with stage (FIGO 1988) IA to IB grades 1 and 2 endometrioid adenocarcinoma were randomized after surgery to observation (n = 326) or intravaginal RT (n = 319). Surgery consisted of TAH/BSO (laparoscopic surgery was allowed), pelvic washing, and removal of enlarged nodes. The dose and type of intravaginal RT varied among the six centers participating in this trial, but 347 of 645 patients were treated with high dose rate (HDR) to 18 Gy in six fractions. The proximal upper two-thirds of the vagina was treated with the dose prescribed to 0.5 cm from the surface of the cylinder. The rate of vaginal recurrence was 3.1% in the observation arm compared to 1.2% for the intravaginal RT arm (p = .114). The rate of pelvic recurrence was 0.9% in the observation arm and 0.3% in the treatment arm (p = .326). No significance difference was seen between the two arms in terms of overall survival. There was significantly more grade 1 vaginal toxicity with intravaginal RT (8.8% vs. 1.5%; p = .00004). There was no significant difference in gastrointestinal (GI) or genitourinary toxicity.
Pelvic RT Versus Intravaginal RT
In the PORTEC-2 trial, 427 patients were randomized to pelvic RT (n = 214) or intravaginal RT (n = 213). Patients enrolled were those with stage (FIGO 1988) IB grade 3 and >60 years old, IC grades 1 and 2 and >60 years old, and IIA grades 1 and 2 of all ages but with <50% myometrial invasion. During surgery patients underwent TAH/BSO, pelvic washing, and removal of suspicious pelvic or para-aortic lymph nodes. Routine lymphadenectomy was not performed. The dose of pelvic RT was 46 Gy given in 23 fractions. Intravaginal RT was delivered using a cylinder to treat the upper half of the vagina. The dose was prescribed to 0.5 cm from the surface of the cylinder. Three types of brachytherapy were used: HDR to 21 Gy in three fractions, low dose rate to 30 Gy at 0.5 to 0.7 Gy/hr, and medium dose rate to 28 Gy at 1 Gy/hr. With a median follow-up of 36 months, the 3-year vaginal recurrence rates were 0.9% in the intravaginal RT arm and 1.9% in the pelvic RT arm (p = .97). The pelvic recurrence was significantly different; the 3-year rate was 3.5% in the intravaginal RT arm compared to 0.6% in the pelvic RT arm (p = .03). The corresponding rates of isolated pelvic recurrence, however, were not significant: 0.6% versus 1.2%, respectively (p = .54). There was no significant difference in disease-free or overall survival between the two arms. The rate of grades 1 and 2 acute GI toxicity was 53% versus 12% in favor of intravaginal RT (p < .001). This trial showed that intravaginal RT alone is sufficient to control vaginal recurrence even in patients with intermediate- to high-risk features.109
In a more recent Swedish trial reported by Sorbe et al.110 patients with stage (FIGO 1988) I endometrioid adenocarcinoma with at least one of the risk factors grade 3, ≥50% myometrial invasion, or DNA aneuploidy were randomized to adjuvant intravaginal RT (IVRT; n = 263) or pelvic and IVRT (n = 264). Lymphadenectomy was required. There was no difference in vaginal recurrence, which was 2.7% (7 of 263) in the IVRT-alone arm compared to 1.9% (5 of 264) in the combined arm (p = .555). Pelvic recurrence rate, however, was different: 5.3% in the IVRT arm compared to 0.4% in the pelvic plus IVRT arm (p = .0006). There was no significant difference in overall survival between the two arms (90% vs. 89%, respectively). The toxicity was significantly higher in the combined arm compared to IVRT alone.
Radiation Treatment Recommendations for Early-Stage Disease Based on Risk Factors
Based on the results of these trials in early-stage endometrial cancer, it is clear that pelvic RT is an excessive treatment for most of those patients. Therefore the treatment recommendations should be individualized based on risk factors. When deciding on whether to recommend observation, intravaginal RT, or pelvic RT, the risk of vaginal recurrence and pelvic recurrence should be assessed separately. With respect to vaginal recurrence, the data from randomized trials indicate that adjuvant intravaginal RT alone is sufficient to control potential microscopic disease in the vagina. The PORTEC-2 trial showed that intravaginal RT is as good as pelvic RT in controlling vaginal recurrence (0.9% vs. 1.9%, respectively; p = .97) despite the fact that patients included in this trial were at high risk for vaginal recurrence based on age ≥60 years old, deep myometrial invasion, or endocervical gland involvement.109 The data from a recent Swedish randomized trial further confirmed that when it comes to vaginal control, IVRT alone is sufficient.110 The vaginal recurrence was 2.9% in the IVRT arm compared to 1.9% (p = .555) in the pelvic plus intravaginal RT arm. How best to reduce pelvic recurrence is more controversial. For patients at low risk of having pelvic lymph node involvement, that is, endometrioid grade 1 or 2 with no or minimal myometrial invasion, neither lymphadenectomy nor pelvic RT is likely to be of significant benefit.111 Those who are at higher risk of having lymph node involvement may need to have their lymph nodes surgically assessed to ensure that they are pathologically negative or receive pelvic RT to control potential microscopic disease. However, the two PORTEC trials, as well as the Swedish trial, showed that the risk of pelvic recurrence was only 2% to 6% even in the absence of lymphadenectomy.104,109,110 This low rate of pelvic recurrence, coupled with the lack of survival advantage to lymphadenectomy and pelvic RT, raises the question of whether either approach is needed for the majority of patients with early-stage endometrial cancer. In the eyes of many, having LVI is almost indicative of nodal involvement. Cohn et al.112 correlated LVI and the risk of positive pelvic nodes in 366 surgically staged patients. The rate of LVI was 25%, and the rate of positive pelvic nodes was 13%. Patients with LVI were significantly more likely to have nodal metastasis (35 of 92 vs. 11 of 274; p < .001). However, the influence of LVI on pelvic nodal metastasis was the strongest in patients with deep myometrial invasion and high grade. Data from MSKCC on 126 patients with endometrioid FIGO (1988) stages IB to IIB and LVI also showed that the mere presence of LVI should not be a trigger for giving pelvic RT, especially when patients had lymphadenectomy. Patients were divided into two groups: those from the old era, when treatment was often pelvic RT, and those from the modern era, when patients were more often treated with lymphadenectomy and intravaginal RT.113 The rate of pelvic relapse for patients with LVI was 7% in the old era compared to 3% (p = .3) in the modern era.
No Myometrial Invasion, Grades 1 and 2
The risk of vaginal recurrence is almost negligible. Straughn et al.114 reported no vaginal recurrence in 103 such patients treated with surgery alone. Pelvic lymph nodal positivity was ≤3%. The 5-year progression-free survival rate in this group was on the order of 95% to 98%. It is unlikely that postoperative pelvic or intravaginal RT would add anything to the final outcome, and therefore radiation is not routinely recommended to this group of patients.
No Myometrial Invasion, Grade 3
In GOG study 33, there were only eight patients with stage IA grade 3 disease, making it difficult to draw any meaningful conclusion.87 There were no relapses in the three patients receiving postoperative radiation as compared with one failure in the five patients who received no postoperative therapy. Straughn et al.114 reported on eight patients with stage IA grade 3 disease treated with surgery alone, with two of patients developing isolated vaginal recurrence. The risk of lymph node metastasis in this group of patients is not very high. At MSKCC, these patients are offered either intravaginal RT alone or observation.
Less Than 50% Myometrial Invasion, Grades 1 and 2
This group of patients constitutes the most common stage subgroup of all endometrial cancers. Straughn et al.114 reported a 3% (9 of 296) risk of vaginal recurrence when surgery alone was done. In the surgery alone arm of the PORTEC-1 trial115 the 5-year vaginal recurrence rate for patients with <50% myometrial invasion grade 2 was 5%. In a randomized trial reported by Sorbe et al.108 the vaginal recurrence rate was 3.1% for those in the observation arm compared to 1.2% for those in the intravaginal RT arm (p = .114). The trial was designed to detect a difference of 1% versus 5% in the vaginal recurrence rate in the two groups. The data were not reported separately based on whether myometrial invasion was present or not, making it difficult to determine the true impact of intravaginal RT on the rate of vaginal recurrence in patients with myometrial invasion. Data from MSKCC on 233 patients with <50% myometrial invasion grade 1 or 2 showed a vaginal recurrence rate of only 1% using intravaginal RT alone.116 In addition, Sorbe et al.117 reported on 110 patients with IB grade 1 or 2 who were part of a prospective, randomized trial evaluating two different intravaginal RT doses; the rate of vaginal recurrence was 0.9%. The risk of pelvic recurrence was only 1.8%, even though lymphadenectomy was not required. This low rate of pelvic recurrence is similar to those reported by Straughn et al.114 of 0.3% (1 of 296) and by Horowitz et al.118 of 0% (0 of 62) in the setting of lymphadenectomy. This indicates that pelvic RT is of limited use, and therefore it seems reasonable to suggest that either observation or intravaginal RT is a reasonable option for patients with grade 1 or 2 and <50% myometrial invasion.
However, when deciding on whether adjuvant RT is needed, it is important to address two issues. First, older patients tend to have higher rates of relapse. In the study by Straughn et al.114 8 of the 10 vaginal/pelvic recurrences were in patients ≥60 years old. In the randomized trial by Sorbe et al.108 comparing adjuvant intravaginal RT to observation, patients with vaginal recurrences were significantly (p = .018) older (mean age, 68.6 years) than patients without vaginal recurrences (mean, 62.6 years). Second, patients with LVI have a higher chance of vaginal recurrence, as demonstrated by Mariani et al.,81 who reported on 508 patients with endometrial cancer limited to the corpus treated with surgery alone. The presence of LVI significantly increased the vaginal relapse rate from 3% to 7% (p = .02). The rate of vaginal relapse would have been even higher if patients without myometrial invasion (152 of 508) had been excluded because LVI is exceedingly rare in patients without myometrial invasion. At MSKCC, patients who are ≥60 years old or have LVI are recommended to have intravaginal RT.
Greater Than 50% Myometrial Invasion, Grade 3
Some advocate observation for patients with <50% myometrial invasion grade 3, yet the 5-year vaginal recurrence rate in PORTEC-1 was 14% for such patients treated with surgery alone compared to 0% for those treated with pelvic RT.115 Perhaps a better choice for those patients is intravaginal RT. The incidence of positive pelvic lymph nodes at time of surgery in this subset of patients is not negligible. In the GOG 33 study the rate was 9% (5 of 54 of patients with inner one-third myometrial invasion), and in the study by Chi et al.119 the rate was 7% (3 of 42) based on <50% myometrial invasion.71 Yet the rate of pelvic recurrence, when the pelvic nodes are not surgically assessed, does not reflect these incidences. In the PORTEC-1 trial, none of the 37 patients with grade 3 disease and <50% myometrial invasion who were treated with TAH/BSO alone relapsed in the pelvis.115 Horowitz et al.118 (n = 31) and Fanning120 (n = 21) reported no vaginal or pelvic recurrence in their series of patients with <50% myometrial invasion grade 3 treated with hysterectomy and lymphadenectomy followed by intravaginal RT. At MSKCC, intravaginal RT is recommended for this subset of patients irrespective of whether lymphadenectomy was performed.
TABLE 70.4 OUTCOME FOR ENDOMETRIAL CANCER WITH ≥50% MYOMETRIAL INVASION (GRADES 1 AND 2) AFTER LYMPHADENECTOMY AND INTRAVAGINAL RADIOTHERAPY ALONE





