Visit the Endocrine Hypertension: From Basic Science to Clinical Practice , First Edition companion web site at: https://www.elsevier.com/books-and-journals/book-companion/9780323961202 .


Introduction
Endocrine disorders in pregnancy which may precipitate hypertension, such as primary aldosteronism (PA), pheochromocytoma, or Cushing syndrome (CS), are uncommon [ ]. Causes which are much less frequently observed may include primary hyperparathyroidism and acromegaly. However, these disorders are associated with a rather significant quantum of maternal and fetal morbidity and mortality. Both in the general population as well as among pregnant women, the diagnosis of hypertension is being made with greater frequency than in the past [ ].
Pregnancy is associated with physiological changes in the hypothalamo-pituitary-adrenal (HPA) axis with a resultant increase in cortisol, renin, and aldosterone levels but with no alteration in the catecholamine levels. This makes the diagnosis of primary hyperaldosteronism and CS challenging during pregnancy. On the other hand, it may be relatively more straightforward to make a diagnosis of pheochromocytoma during pregnancy, if a high index of clinical suspicion is maintained [ , ]. This chapter details the clinical, diagnostic, and therapeutic approaches to endocrine hypertension in pregnancy.
Primary hyperaldosteronism in pregnancy
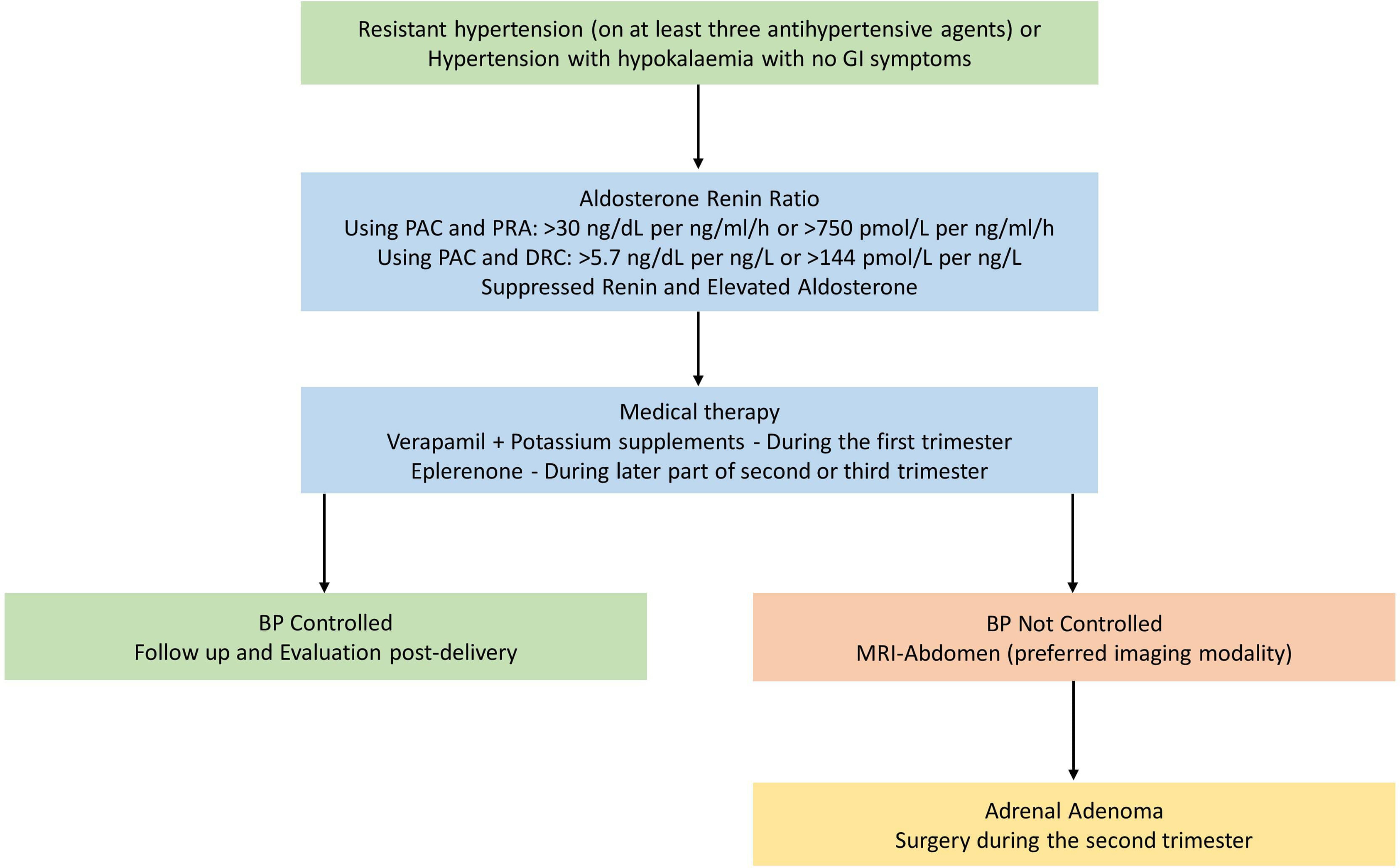
In a pregnant woman presenting with resistant hypertension or hypertension with low serum potassium levels without vomiting, primary hyperaldosteronism should be considered as one of the differential diagnoses in addition to gestation-related hypertension. However, one should remember that more than half of all patients with primary hyperaldosteronism might present with normal serum potassium levels [ ]. Therefore, resistant hypertension with normokalaemia in a young patient warrants an evaluation for primary hyperaldosteronism.
During pregnancy, there is a significant physiological alteration in the blood volume due to an increase in renin and aldosterone levels as well as atrial natriuretic peptide levels. However, hypokalemia does not occur despite having high renin and aldosterone levels [ , ]
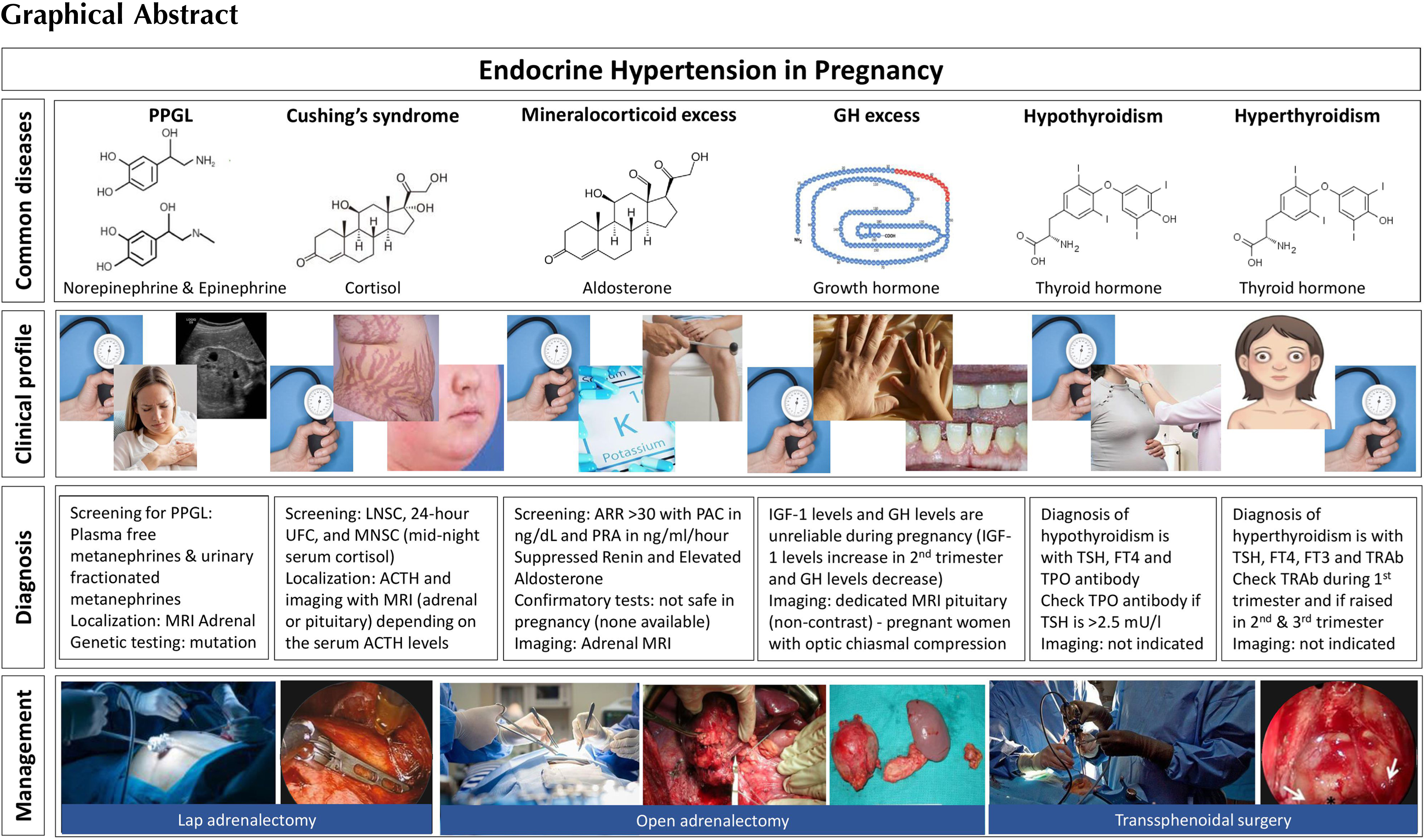
Renin and aldosterone levels rise in pregnancy, causing an alteration in the aldosterone and renin ratio (ARR). This change was observed as early as the sixth week of gestation. During pregnancy, renin is generated in the kidney and placenta. The factors that regulate renin from the placenta are not clearly understood. Though there is an increased level of angiotensin II, this is countered by a reduction in responsiveness to angiotensin II. Moreover, aldosterone levels may increase up to 10 times greater than the normal limit, toward the end of gestation [ ]. Despite all these hormonal alterations, an intact renin-angiotensin-aldosterone system (RAAS) is maintained during pregnancy. Progesterone plays an important role in potassium homeostasis, which acts as a competitive aldosterone inhibitor on the mineralocorticoid receptor, thereby maintaining normal plasma potassium levels in pregnant women despite having activated the RAAS [ ].
A previous history of periodic paralysis that has been associated with hypertension also helps in suspecting the clinical diagnosis of primary hyperaldosteronism. A lowered serum potassium with an elevated bicarbonate level, without an associated gastrointestinal loss of potassium, provides clues to the diagnosis [ ]. Since there is a physiological alteration in the angiotensin–renin–aldosterone axis, a biochemical diagnosis of primary hyperaldosteronism should be done diligently following serial evaluation.
A challenge that may be encountered in elucidating the diagnosis is that there are no large-scale studies that clearly define the ARR cutoff values in pregnant women with primary hyperaldosteronism. Studies have shown that the nonpregnant reference interval for the diagnosis of primary hyperaldosteronism in itself is adequate for the diagnoses of primary hyperaldosteronism [ ].
Definitive diagnostic tests such as the saline loading test, captopril test, or fludrocortisone test may not be appropriate to conduct in pregnant women, as they have not been clearly studied and there are certainly concerns with their safety during pregnancy. Once the diagnosis is confirmed, the subsequent challenge is to choose the appropriate imaging modalities to confirm the diagnosis of primary hyperaldosteronism [ ]. Due to the lack of radiation risk, magnetic resonance imaging (MRI) of the abdomen may be preferred over a computed tomography (CT) scan during pregnancy. In pregnancy, the prevalence of an adrenal adenoma is far more common than that of bilateral adrenal hyperplasia [ ]. The therapy of hyperaldosteronism due to bilateral adrenal hyperplasia involves the usage of mineralocorticoid receptor antagonists such as spironolactone or eplerenone. However, spironolactone may potentially cause feminization of the male fetus [ ].
Therefore, therapy with calcium channel blockers such as verapamil may be a safe alternative for blood pressure control along with oral potassium supplements in patients with primary hyperaldosteronism. By and large, none of the antihypertensive agents which are commonly used, may be categorized as safe in pregnancy, particularly with reference to the first trimester. The medications that may be of use during pregnancy are shown in Table 19.1 .
| Medications | Category | Dose (as divided doses) | Dosage intervals |
|---|---|---|---|
| Alpha methyldopa | B | 500 mg–3 g | Twice to thrice daily |
| Labetalol | C | 200–1200 mg | Twice to thrice daily |
| Nifedipine | C | 30–120 mg | Twice to thrice daily |
| Hydralazine | C | 50–300 mg | Twice to thrice daily |
| Beta blocker | C | Dosage depends on the drug | Once (sustained-release) to twice daily |
| Hydrochlorothiazide | C | 12.5–50 mg | Once daily |
During the second trimester, patients may be given eplerenone, which is a safe alternative for spironolactone owing to its selective mineralocorticoid receptor antagonist property without a feminisation effect [ ]. However, in situations wherein inadvertent usage of spironolactone has been reported, feminization of the male fetus has not been observed.
Patients with Conn’s syndrome having a definite adrenal adenoma may need surgical excision during the latter half of the second trimester. Adrenal adenomas that are diagnosed during early pregnancy should be treated with antihypertensives and potassium supplements until the end of the second trimester by which time surgery may be relatively safe for mother and fetus [ ].
During the postoperative phase, nearly 5% of patients have mineralocorticoid deficiency, and therefore close monitoring of these patients with serum sodium and potassium is required after surgery. Persistent hypotension during the postoperative period is a marker for mineralocorticoid deficiency. These patients might require a short duration of mineralocorticoid replacement [ ].
A few may require antihypertensive agents even after surgery, particularly if they have end-organ damage in the form of proteinuria, severe hypertensive retinopathy, or left ventricular hypertrophy. Therefore, evaluation for end-organ damage will provide predictive clues for the persistence of hypertension following adrenalectomy [ ].
An algorithm for evaluation and management of primary hyperaldosteronism is shown in Figure 19. 1
Retroperitoneal approach through laparoscopy is considered to be safe in pregnancy for the resection of adrenal tumours [ ].
For more detailed reading on primary hyperaldosteronism, please refer Chapter 21, Chapter 7, Chapter 8 .
Cushing syndrome in pregnancy
Patients with CS may have associated suppression of gonadotrophins, and thereby they often have menstrual irregularities which may subsequently result in anovulation [ ]. Therefore, infertility is common among patients with CS. However, patients with CS may present for the first time with isolated severe hypertension during pregnancy.
Untreated CS increases the risk of spontaneous abortion, perinatal death, premature birth, intrauterine growth retardation, hypertension, preeclampsia, and diabetes mellitus [ ]. Adrenal adenomas are the causes of CS in up to 50% of pregnant women with endogenous CS, in contrast to only 15% of endogenous CS in nonpregnant women [ ].
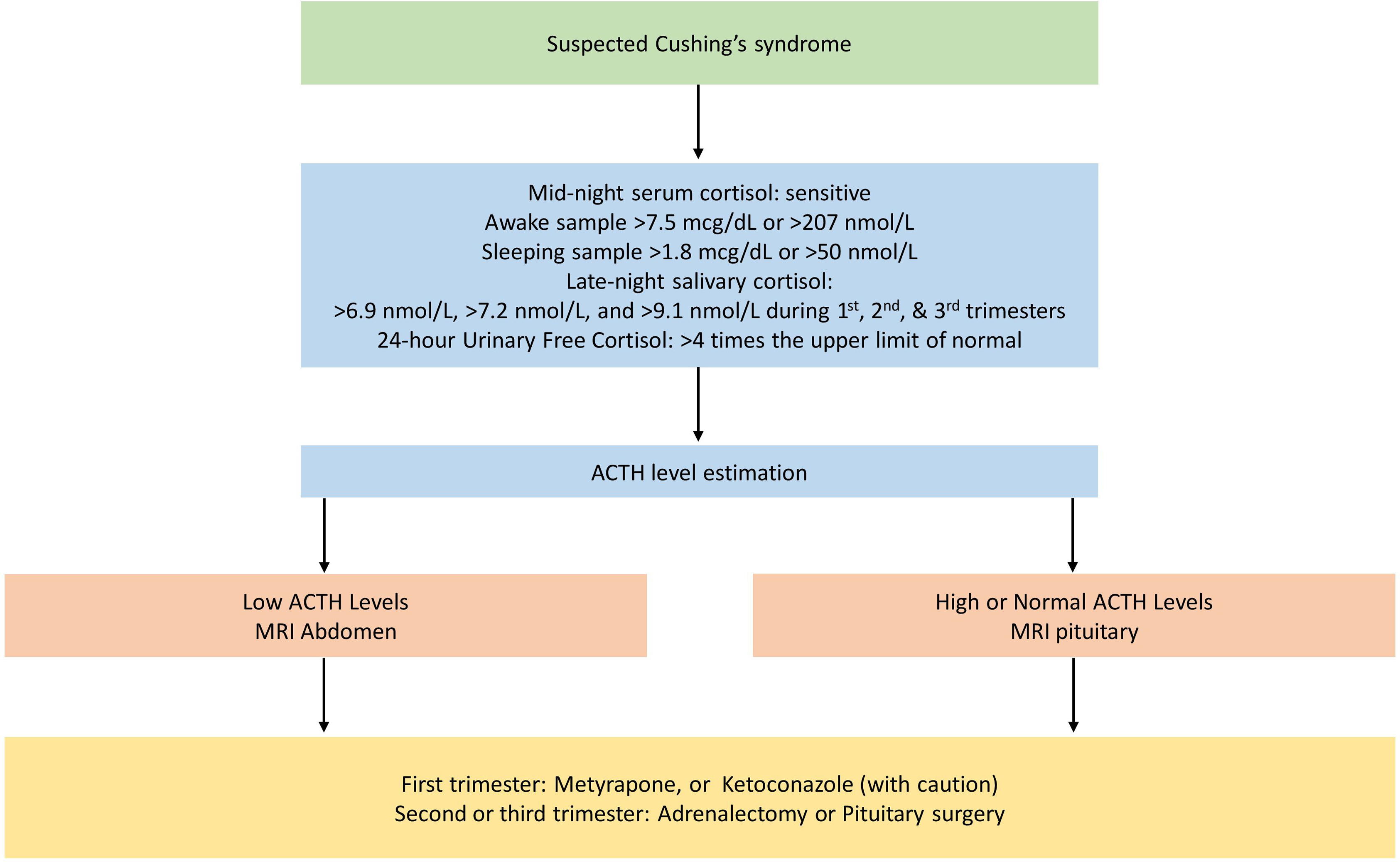
Unlike patients with primary hyperaldosteronism, patients with endogenous CS may have specific clinical features such as weight gain, hirsutism, emotional lability, and acne which may also be occasionally seen in normal pregnant women. However, a triad of hypertension, ecchymosis, and proximal muscle weakness in a pregnant woman should raise the suspicion of CS [ ].
Once clinically suspected, the diagnosis may include a confirmation of altered circadian rhythm with a mid-night sleeping serum cortisol of more than 1.8 mcg/dL (50 nmol/L) or awake serum cortisol of more than 7.5 mcg/dL (207 nmol/L). Often, the effect of one milligram overnight dexamethasone suppression test response on the 8.00 am serum cortisol may be blunted and is therefore largely unreliable. This effect may be commonly observed during the second and third trimesters of pregnancy [ , ]. Moreover, the 24-hour urine free cortisol may be also misleading, as it may be up to threefold high among normal pregnant women resulting from an increase in the circulating cortisol. The high-dose dexamethasone suppression test also usually shows a variable result [ ].
However, a quadrupling of elevated urinary free cortisol levels may be used alongside other confirmatory tests for the diagnosis of CS in pregnancy. In addition, the ACTH levels can be non-suppressed even in patients with an adrenal adenoma due to the impact of placental CRH on the maternal pituitary gland [ ]. Recent evidence suggests that threefold the upper limit of normal late-night salivary cortisol or LNSC (>12 nmol/L; the normal upper limit of late-night salivary cortisol being 145 ng/dL or 4 nmol/L) has a 100% sensitivity and specificity for the diagnosis of overt CS in nonpregnant subjects [ ]. Another recent study has shown that the same late-night cortisol cutoffs as healthy women can be used in pregnant women and women on oral contraceptives [ ]. The LNSC threshold values for each trimester of pregnancy has been defined: <6.9 nmol/L for the first trimester, <7.2 nmol/L for the second trimester, and <9.1 nmol/L for the third trimester [ ]. The LNSC is considered the most robust criterion for a positive diagnosis of CS during pregnancy [ ]. More sophisticated evaluation with the statistical tools such as ROC curves, and likelihood ratios are not available for these tests during pregnancy.
An MRI without gadolinium is the most appropriate imaging modality for the localization of hypercortisolism in pregnancy [ ]. However, an MRI pituitary without gadolinium might not detect a microadenoma. Moreover, the size of the pituitary increases during pregnancy, and the radiological difference between a pathological lesion and physiological enlargement may not be clearly discernible [ ]. If an adrenal adenoma is suspected, an MRI of the abdomen is the imaging modality of choice to localize the lesion.
In patients with a proven hypercortisolemic state, medical or surgical therapy should be individualized to reduce the risk of fetal loss. Treating the other comorbidities such as diabetes and hypokalaemia is also important. Hypertension in patients with CS and pregnancy is treated with calcium channel blockers such as nifedipine or amlodipine, or a centrally acting medication such as methyldopa [ ].
Typically, the timing of surgery for both adrenal adenomas and pituitary microadenomas should be delayed to the later part of the second trimester. Though there is no wide experience or case series available in this regard, a first-trimester surgical intervention is better avoided, as it may result in pregnancy loss [ ].
If CS is diagnosed during early pregnancy, metyrapone (250–750 mg given in three divided doses per day), an inhibitor of 11β-hydroxylase in the steroidogenesis pathway of the adrenal cortex, is the medication of choice in reducing the serum cortisol to the acceptable levels for pregnancy. Metyrapone may cross the placenta, but its effect on fetal steroid production is rather negligible. Metyrapone may also cause hypertension and hypokalemia at higher doses owing to the accumulation of 11 deoxycorticosterone. Very occasionally hypocortisolemia may occur with the prolonged usage of metyrapone [ ]. Therapy with ketoconazole is limited for use in patients with severe hypercortisolemia, due to its theoretical effect on potentially causing feminization of a male fetus, and maternal hepatotoxicity. However, in practice, feminization does not occur [ ]. Other drugs such as mitotane, etomidate, and mifepristone that are used for hypercortisolism have severe teratogenic effects and are not studied extensively among pregnant women [ ].
If metyrapone is continued throughout pregnancy, it should be temporarily withdrawn prior to labour, to maintain normal cortisol production. The therapies for the hypercortisolemic state should be restarted following childbirth. In addition, all patients on medical management, as well as those who achieve surgical remission should receive intravenous hydrocortisone during the intrapartum phase. Hydrocortisone can be gradually tapered and subsequently stopped [ ].
An algorithm for diagnosis and management of CS during pregnancy is shown in Fig. 19.2 .
For further reading on CS, please refer Chapter 13, Chapter 14, Chapter 21 .
Pheochromocytoma and paraganglioma (PPGL) in pregnancy
Though unusual, pheochromocytoma may be a cause of secondary hypertension in pregnant women presenting with hypertension. Often, catecholamine secreting tumours cause typical paroxysmal symptoms of palpitations, impending doom, headache, tremors, and diaphoresis. However, both eclampsia and preeclampsia can raise the catecholamine levels, which could be a confounder in biochemical screening for PPGLs [ ]
Episodic hypertension is a good clinical indicator in differentiating pheochromocytoma from other causes of secondary hypertension. There are several genetic mutations that are associated with PPGLs [ ]. Untreated pheochromocytoma induces an increased risk for both the mother and the fetus due to uncontrolled hypertension and not due to elevated maternal catecholamine levels. Repeated cycles of episodic hypertension associated with rebound normotensive episodes may cause placental abruption, and severe hypoxia, thereby resulting in severe fetal distress [ ].
The diagnosis in pregnant patients is often missed, as symptoms due to pheochromocytomas can mimic gestational hypertension or preeclampsia. However, preeclampsia is associated with proteinuria and pedal oedema, as well as sudden weight gain with associated biochemical (liver) or coagulation abnormalities. Moreover, obesity is an important risk factor that may contribute to preeclampsia. The differential diagnosis of a catecholamine secreting tumour should be considered in a pregnant woman presenting with a history of labile hypertension, headaches, palpitation, and the failure to respond to conventional antihypertensive agents. New-onset orthostatic hypotension is considered to be an important symptom in pregnant women with a pheochromocytoma. Other notable symptoms are related to cardiovascular manifestations, such as arrhythmias, acute heart failure, and cardiogenic shock [ ].
Elevated 24-hour urinary fractionated metanephrine levels may suggest the diagnosis of PPGL and warrant an imaging evaluation. Plasma-free metanephrine levels may be performed in certain centres [ ]. However, due to the unstable nature of metanephrines in usual laboratory conditions, it is not been frequently utilized at present in smaller centers [ , ]. An ultrasonography (USG) of the abdomen may be an initial screening modality; however, an enlarged uterus might make intraabdominal structures difficult to visualize in the case of an adrenal or a small extra-adrenal lesion. MRI of the abdomen is the most suitable imaging modality for localizing an adrenal or extra-adrenal mass during pregnancy.
Once a diagnosis is confirmed, the patient should be adequately hydrated (with at least 4–5 L of fluids per day), and salt loaded (with 8–12 g of NaCl a day) along with selective alpha-adrenoceptor blockade with drugs like prazosin or doxazosin for a period of 10–14 days. A nonselective alpha-blocker such as phenoxybenzamine might also be used; however, it should be reserved for life-threatening hypertension, as phenoxybenzamine may cross the placenta and could cause hypotension in the neonate. Moreover, it could induce perinatal depression in the mother. Prazosin and doxazocin are both safe alternatives to phenoxybenzamine. Beta-adrenergic blockers should be started preoperatively following adequate alpha-adrenergic blockade to prevent perioperative arrhythmia risk [ ]. Beta-adrenergic blockers should be initiated prior to surgery to keep the heart rate less than 90/min. Esmolol, a cardio-selective β1 adrenergic receptor antagonist, or metoprolol (category-C) can be used as a beta-adrenergic blocking agent. Some studies also suggested that labetalol, a mixed α and β receptor antagonist, is a safe alternative owing to its short duration of action. In patients with preeclampsia, magnesium sulphate is a safe medication and is of value in preventing further progression to eclampsia. Magnesium sulphate inhibits the catecholamine release, induces the peripheral catecholamine receptor level blockade, and is a direct vasodilator. Other medications that are effective in the setting of a hypertensive crisis are hydralazine, nitroglycerine, and sodium nitroprusside. Due to the short-acting nature of esmolol and labetalol, both these medications can be used in hypertensive crises in the presence of tachyarrhythmias [ ].
Surgery is the definitive treatment for a pheochromocytoma. There are no definitive guidelines for the timing of surgery in pregnancy. However, the timing depends on the gestational age, the accessibility of the tumour, and the presence of foetal distress. According to the literature, the tumour removal can be done in early pregnancy following adequate alpha- and beta-adrenoceptor blockade [ ]. If the diagnosis is made after 24 weeks of gestation, surgical excision is recommended along with a caesarean section. Normal vaginal delivery has been shown to be associated with a higher maternal mortality rate in comparison with a caesarean section [ ].
Fig. 19. 3 . shows an algorithm for evaluation and management of pheochromocytoma/paraganglioma during pregnancy.

Most patients do not require postoperative antihypertensives and remain normotensive afterwards [ ]. All patients with pheochromocytomas should be screened for genetic causes for PPGL syndromes such as multiple endocrine neoplasia 2a and 2b (MEN2a/MEN2b), von Hippel-Lindau (VHL), or SDH mutation related syndromes. Moreover, patients with a PPGL syndrome should be followed up periodically for the tumour recurrence as well as other manifestations of the genetic mutations.
Please refer to Chapter 2, Chapter 10, Chapter 11, Chapter 21 for additional reading about PPGLs
Primary hyperparathyroidism
Primary hyperparathyroidism very rarely presents with hypertension as an initial manifestation in pregnant women with a reported prevalence of ∼0.9% in the general population. The calcium requirement for the developing fetus increases to a peak level toward the end of the third trimester. During the initial two trimesters, due to hemodilution and decrease in the albumin concentration, the serum calcium levels drop to low normal levels, but with no change in the ionised calcium or phosphate levels. An increase in calcium requirements during the third trimester is augmented by an increase in the parathyroid hormone related polypeptide (PTHrP) levels which may be secreted by various maternal organs. The calcium levels in the developing fetus are maintained by maternal as well as its own circulating serum calcium [ ].
The maternal complications associated with hypercalcemia include nephrolithiasis, pancreatitis, and pre-eclampsia. Severe hypercalcemia may also cause vomiting and dehydration, thereby aggravating hyperemesis gravidarum. The fetal complications due to hypercalcemia include intrauterine growth restriction, preterm delivery, low birth weight, neonatal hypocalcemia, tetany, and an increased risk of miscarriage. Patients with primary hyperparathyroidism in pregnancy, in general have a past history suggestive of fractures or renal stones, if the hypercalcemia is very severe [ ]. A minority of pregnant women with primary hyperparathyroidism may have worsening hypercalcemia during pregnancy due to the effect of PTHrP, and this could potentially precipitate a hypercalcemic crisis [ ]. Significant hypertension associated with primary hyperparathyroidism is rather uncommon or rare, and is generally not severe. Biochemical diagnosis of hyperparathyroidism may be associated with hypercalcemia, hypercalciuria, and phosphaturia with elevated parathormone levels. Ultrasonography of neck is the imaging modality of choice for the localization of a parathyroid adenoma. Nuclear scans such as parathyroid scintigraphy and other imaging modalities like 4D CT are contraindicated in pregnancy [ ].
The conservative management may largely encompass hydration and avoiding calcium supplementation. The level of calcium at which surgery should most certainly be advocated is still not clear due to a paucity of data [ ]. Bisphosphonates should be avoided due to their teratogenic effects on the fetus [ ]. Limited evidence suggests that cinacalcet and other calcimimetic agents may be used with caution in cases of severe hypercalcemia [ ]. The Second trimester is the most appropriate time to plan for parathyroidectomy [ , ]. Postoperative transient hypocalcemia should be managed with at most care, as it may precipitate hypercalcemia in the fetus. Hence, therapy with calcium and activated vitamin D should be initiated if postoperative hypocalcemia is detected.
Though a majority of patients with primary hyperparathyroidism (up to 90%) occur sporadically, the remaining patients may have an associated genetic cause for parathyroid adenoma, that includes MEN1, MEN2A, MEN4, hyperparathyroidism jaw tumour (HPT-JT) syndrome, and familial isolated primary hyperparathyroidism (FIPH) [ ]. Therefore, genetic testing in pregnant women should be considered for syndromic causes for primary hyperparathyroidism as most patients present at an early age with hypercalcemia [ ].
Please refer Chapter 16, Chapter 21 for additional reading on this topic.
Hyperthyroidism and hypothyroidism in pregnancy
Pregnancy and its impact on the thyroid have been well studied [ ]. The fetal thyroid gland functions from 18 to 20 weeks of gestation, and until then the fetus mostly depends on maternal thyroxine. Physiological changes of pregnancy such as an increase in the concentration of thyroxine–binding globulin (TBG) due to a hyper-estrogenic state, increased urinary iodide clearance, and an increase in thyroxine degradation by type 3 deiodinase in the placenta occur during pregnancy. However, higher concentrations of human chorionic gonadotropin (hCG), which is a weak agonist of the TSH receptor, induces an increase in thyroxine production [ ].
Thyroid hormone-related disorders are common in pregnancy. However, those disorders which present as hypertension in pregnancy are very rare. Poorly controlled or untreated thyrotoxicosis precipitates miscarriages, gestational hypertension, prematurity, low birth weight, intrauterine growth retardation in the fetus, and thyroid storm as well as congestive heart failure in the mother. Studies have shown that uncontrolled thyrotoxicosis during pregnancy increases the risk of preterm delivery and severe pre-eclampsia by 16 and 5 fold, respectively [ ].
The diagnosis of over hyperthyroidism includes suppressed TSH and elevated free and total thyroxine levels. One should keep in mind that the pregnant women can have TSH in the low normal range and total thyroxine up to 1.5 times the upper limit of normal owing to the physiological changes that have already been described [ ]. The therapy for hyperthyroidism includes therapy with beta-adrenergic blockers for sympathetic overactivity and controlling hyperthyroxinemia with antithyroid medications [ ].
Antithyroid drugs such as methimazole or propylthiouracil are both associated with very occasional adverse effects when used in the early gestational period. Propylthiouracil may rarely be associated with maternal liver injury. The fetal effects include birth defects, such as preauricular sinuses and hydronephrosis. In addition, methimazole or carbimazole may be associated with maternal agranulocytosis and foetal malformations such as choanal atresia, aplasia cutis, and omphalocele [ ]. Both these effects are generally idiosyncratic and could be dose-dependent, and often noticed when used at higher doses. These adverse effects can be avoided by using the lowest possible dose of antithyroid drugs. Using “block and replace” therapy in the form of antithyroid drugs and levothyroxine is not a good option for pregnant women with hyperthyroidism [ , ].
All patients treated with antithyroid drugs should be followed up at least once in 2–3 weeks initially to keep the free thyroxine level (FT4) close to the upper limit of normal. Most often, toward the third trimester, the dose of antithyroid medications need to be reduced to the lowest possible dose or may need to be withdrawn due to associated immune tolerance in the fetus. Large scale studies have shown that the use of propylthiouracil during the first trimester is associated with a lower risk of fetal adverse effects compared to carbimazole. Therefore, switching over to propylthiouracil is needed in women who are already on methimazole. Once the 16-week critical period of organogenesis has been crossed, the patients may be switched over to methimazole. Radio-iodine ablation is contraindicated in pregnant women [ ].
A persistently high TSH receptor antibody (TRab) of more than three times the upper limit of normal during the third trimester is associated with an increased risk of fetal hyperthyroidism [ ]. Therefore, for all the patients with newly detected hyperthyroidism as well as those who have already been treated with antithyroid drugs or are in remission, TRAb should be checked. Patients can be kept on follow-up if the maternal TRAb levels are low or undetectable during the first trimester.
If TRAb is high, TRAb should be repeated between 18 and 22 weeks. Of those with more than 3 times the upper limit, TRAb should be rechecked between 28 and 34 weeks as this poses a high risk for fetal hyperthyroidism [ ].
Thyroidectomy should be reserved for those who are not tolerating antithyroid drugs or requiring a very high dose for control of hyperthyroid symptoms, or have an allergy to antithyroid medications, or those with a large goitre. An algorithm for evaluation and management of hyperthyroidism in pregnancy is shown in Fig. 19.4 .