INTRODUCTION
In the past two decades, cancer research has rapidly advanced, spurred by the development of high-throughput technology and the maturation of genomic and proteomic research methods. These advances have resulted in treatments that have substantial effects on the outcomes of certain cancers. The continuous development of new antineoplastic agents adds increasing challenges for practicing physicians.
Current cancer treatments include surgery, radiation, cytotoxic chemotherapy, hormonal therapy, bio-immunotherapy, and targeted therapy. Adverse effects of antineoplastic agents on the endocrine system are caused by several different mechanisms and can range from a subtle laboratory abnormality with limited clinical significance to potentially lethal clinical syndromes. Antineoplastic agents in general can be cytotoxic to endocrine cells and result in glandular dysfunction. Antineoplastic agents can also interfere with the synthesis or postsynthesis processing of hormones at different levels (ie, transcription, translation, or posttranslation). An agent may inhibit or induce secretion of a hormone by interacting with receptors, perturbing intracellular second messenger metabolism, or may affect hormone delivery by changing carrier protein levels in serum or by competing for binding on the carrier protein. Finally, antineoplastic agents can interact with signal transduction pathways to inhibit or enhance hormonal action in the end organs.
In this chapter, we summarize the major and common endocrine complications of cancer therapy and discuss screening and surveillance of these complications in cancer patients and survivors.
METABOLIC DISORDERS
Serum glucose is under continuous complex regulation. Many processes can affect glucose levels, including gut absorption, cellular uptake, gluconeogenesis, and glycogenolysis. Multiple hormones also play important roles in overall glucose homeostasis, including insulin, glucagon, growth hormone (GH), cortisol, somatostatin, and incretins.
Glucocorticoids are frequently used with many chemotherapy protocols and can have profound effects on glucose levels by increasing insulin resistance. Glucocorticoids can unmask preexisting prediabetic states by precipitating overt diabetes or make diabetes more difficult to control. The severity may range from asymptomatic hyperglycemia to nonketotic hyperosmolar coma. Most patients taking glucocorticoids with elevated glucose require insulin therapy to achieve blood glucose control, especially when given high-dose steroids. Long-acting and intermediate-acting insulin formulations are often combined with mealtime rapid-acting or short-acting insulins. Currently, there emerging studies about the management of steroid-induced diabetes mellitus in cancer patients by using multiple daily injections including mealtime short-acting insulin to counteract postprandial glucose excursions. Recent concerns about the promotion of malignancy by the mitogenic effect of insulin (1) and especially insulin analogs (2) that cross-activate insulin-like growth factor 1 (IGF-1) receptors (3), in combination with conflicting clinical study results on insulin glargine and cancer, have brought attention to the gap in knowledge about proper diabetes management for maximization of survival in cancer patients and survivors. A large cohort study showed that insulin analogs including insulin glargine are associated with a lower risk of cancer in general than human insulin (4).
Mammalian target of rapamycin (mTOR) inhibitors, L-asparaginase, streptozocin, and interferon-α (IFN-α) have also been associated with impaired glucose homeostasis and frank diabetes mellitus (5).
Phosphoinositide-3 (PI3) kinase/Akt/mTOR pathway–targeted therapy can cause hyperglycemia. Inhibition of this pathway results in peripheral insulin resistance, increased gluconeogenesis, and hepatic glycogenolysis (5). Everolimus, a tyrosine kinase inhibitor, is used in patients with advanced breast cancer, progressive neuroendocrine tumors of pancreatic origin, and advanced renal cell carcinoma. Fifty percent of patients taking everolimus have hyperglycemia (Table 52-1) (6). Temsirolimus is another kinase inhibitor used in patients with advanced renal cell carcinoma. The incidence of hyperglycemia in patients using this drug is 26% (7). The mechanism by which temsirolimus leads to diabetes may be similar to that of tacrolimus, which decreases glucose-stimulated insulin release in the pancreatic islets by reducing adenosine triphosphate (ATP) production and glycolysis (8).
| Drug | Type | Mechanism | Main Indications | Endocrine Adverse Effect(s) (rate) |
|---|---|---|---|---|
| Everolimus | Rapamycin analog | Inhibits mTOR | Advanced hormone receptor–positive, HER2-negative breast cancer; progressive neuroendocrine tumors of pancreatic origin; and advanced renal cell carcinoma | Hyperglycemia (50%) Hypercholesterolemia (76%) Hypertriglyceridemia (71%) |
| Temsirolimus | Rapamycin analog | Inhibits mTOR | Advanced renal cell carcinoma | Hyperglycemia (26%) Hypercholesterolemia (24%) |
| Idelalisib | Small-molecule inhibitor | Inhibits PI3 kinase selectively | Chronic lymphocytic leukemia | Hyperglycemia (54%) |
Idelalisib, a PI3 kinase inhibitor, is indicated in patients with chronic lymphocytic leukemia. One of the idelalisib’s emergent laboratory abnormalities is hyperglycemia, which occurred in 54% of patients taking both idelalisib and rituximab in a phase III study (9,10). Another study showed that idelalisib alone in different dose regimens increased serum glucose in 40% of patients (11).
L-Asparaginase is used mainly to treat hematologic malignancies. The risk of hyperglycemia associated with pegylated Escherichia coli asparaginase has been reported to be similar to the risk associated with native asparaginase; in one study, the risk was about 20% in children with acute lymphoblastic leukemia treated with either agent. The exact mechanism of L-asparaginase–associated hyperglycemia is not known, although it has been postulated that inhibition of insulin, insulin receptor synthesis, or both may be the cause, leading to a combined insulin deficiency–insulin resistance syndrome (12). Pancreatitis, which can occur with L-asparaginase therapy, is another possible mechanism for hyperglycemia. Pancreatitis can cause islet cell destruction, and some patients might require insulin therapy (13). One potential complication is hypoglycemia after cessation of L-asparaginase; thus, close monitoring of blood glucose is recommended. Diabetic ketoacidosis has been reported during L-asparaginase therapy. Long-term insulin therapy may not be needed in some cases of L-asparaginase–induced diabetes mellitus (12).
Streptozocin, used primarily to treat malignant islet cell tumors and other neuroendocrine tumors, is an N-nitrosourea derivative of glucosamide. Streptozocin’s effect on islet cells is species specific and dose related; rat islet cells appear to be more susceptible to the cytotoxic effects of streptozocin than human islet cells. Most of streptozocin’s effects are reversible upon discontinuation of the drug. Although the reported incidence of glucose intolerance varies from 6% to 60%, most cases are mild to moderate in severity (14).
Interferon therapy activates immune system cells to fight some cancers and certain infections. According to a survey on IFN therapy in Japan, some patients may experience earlier development of type 1 diabetes, resulting in initiation of insulin therapy (15). These patients were positive for islet cell antibody and anti-glutamic acid decarboxylase antibodies (16).
We recommend monitoring of fasting serum glucose levels prior to the start of and during these therapies and possibly determining the levels of anti-islet autoantibodies before IFN therapy in patients with family history of type 1 diabetes mellitus (15).
Some antineoplastic drugs (eg, ifosfamide and mercaptopurine) cause a proximal tubular defect and lower the renal threshold for glucosuria without affecting glucose metabolism. Glucosuria has been detected with an increased incidence in 67% of adult and 75% of pediatric patients treated with high-dose ifosfamide, cisplatin, and high-dose methotrexate, compared to the early postchemotherapy assessment (13% adults and 29% children) (17).
Lipid disorders are seldom evaluated in the process of active anticancer therapy, because patients are often encouraged to maintain a positive metabolic balance via liberal oral intake. Investigation or treatment of mild lipid abnormalities is often overlooked because the focus is on maintaining a positive caloric balance during cancer treatment. Some lipid disorders may be short-lived without clear clinical consequences, but some may be of clinical importance and need to be detected and treated. In general, triglyceride levels higher than 1,000 mg/dL increase the rate of complications, including pancreatitis.
Lipid disorders are among the main side effects of vitamin A derivatives, which are commonly used in dermatologic disorders. One vitamin A derivative, bexarotene, has been used against malignancies like cutaneous T-cell lymphoma, acute promyelocytic leukemia, and head and neck cancer. Bexarotene is an agonist of retinoid X receptors, a family of peroxisome proliferator-activated receptors that are upregulated by the binding of bexarotene to a receptor on the nucleus. This upregulation not only regulates lipid metabolism but also affects thyroid hormone synthesis (18). Hypothyroidism contributes to lipid disorders in patients receiving bexarotene. Because bexarotene causes hypertriglyceridemia in approximately 40% of patients, lipid levels and thyroid functions should be checked before bexarotene therapy. If triglyceride levels are 200 to 400 mg/dL, dietary modifications are recommended. If triglyceride levels are 400 to 1,000 mg/dL, omega-3 fatty acids with fibrates or nicotinic acid should be started. Lipid levels should be checked after initiation of therapy, because triglyceride levels over 1,000 mg/dL increase the risk of acute pancreatitis (19).
Hypercholesterolemia is the second most common side effect of bexarotene, having been reported in 48% of treated patients (20). The long-term significance of drug-induced hypercholesterolemia is unclear; however, atorvastatin has been successfully used to treat bexarotene-associated hypercholesterolemia in patients at the University of Texas MD Anderson Cancer Center (MDACC).
Mitotane, an analog of the insecticide dichlorodiphenyltrichloroethane, is used in patients with adrenocortical carcinoma as adjuvant therapy. The potential side effects of this therapy include hypercholesterolemia. Although the exact mechanisms of hypercholesterolemia remain unclear, mitotane stimulates hydroxymethylglutarate–coenzyme A reductase. A study from MDACC showed that mitotane increases high-density lipoprotein cholesterol, low-density lipoprotein (LDL) cholesterol, and triglyceride levels (21). Patients with adrenocortical carcinoma usually have a poor prognosis, making the clinical significance of mild to moderate elevation of cholesterol uncertain. However, in long-term survivors on adjuvant mitotane therapy, hyperlipidemia can lead to early development of atherosclerotic disease. The benefits of treating mitotane-induced lipid abnormalities in long-term survivors have not been established.
Mammalian target of rapamycin inhibitors have metabolic side effects that include hypercholesterolemia and hypertriglyceridemia (see Table 52-1). Although the mechanisms of these side effects are unclear, hypercholesterolemia can be caused by dysregulation of sterol regulatory element binding proteins in the mTOR pathway (22). Another possible mechanism is reduction in lipid clearance from the bloodstream (23).
Before starting mTOR inhibitor therapy, baseline fasting glucose, LDL cholesterol, and triglyceride levels should be checked. The lipid profile should be monitored for every cycle. The goals of lipid-reducing therapy are to keep fasting LDL cholesterol at or below 190 mg/dL and triglycerides at or below 300 mg/dL, if life expectancy is >1 year. Therapeutic lifestyle changes are the first appropriate approach for patients with hyperlipidemia. If such lifestyle changes fail to reduce LDL cholesterol to ≤190 mg/dL, statin therapy should be started. The goals of LDL cholesterol reduction vary with patients’ cardiovascular risk factors. Patients with triglyceride levels above 1,000 mg/dL have an increased risk for acute pancreatitis. Fibrate, omega-3 acid esters, niacin, and combination therapy are the treatment options for hypertriglyceridemia (5).
Serum osmolality is tightly regulated, primarily by interaction between the hypothalamic osmoreceptors that regulate secretion of antidiuretic hormone from cells in the paraventricular and supraoptic nuclei, the hypothalamic thirst center, and the kidneys. Disruption of any of these regulators may lead to a disturbance in free water clearance and subsequent abnormalities in serum sodium levels.
Hyponatremia is a relatively common electrolyte abnormality in patients with cancer. The syndrome of inappropriate antidiuretic hormone secretion (SIADH) is one of the most common underlying causes for hyponatremia in this patient population. In addition to its association with hyponatremia, SIADH is characterized by low serum osmolality and an inappropriately high urine osmolality with elevated urine sodium. SIADH is a diagnosis of exclusion after ruling out hypovolemia, heart failure, renal insufficiency, cirrhosis, adrenal insufficiency, hypothyroidism, salt-wasting syndrome, and the use of diuretics. In patients with cancer, SIADH may be caused by ectopic antidiuretic hormone production by a variety of tumors. Syndrome of inappropriate antidiuretic hormone secretion is most commonly seen in patients with small cell lung cancer. Other tumors described (less commonly) in association with SIADH include malignant thymoma, oral squamous cell carcinoma, prostate carcinoma, and pancreatic carcinoma. Chemotherapy-induced lysis of antidiuretic hormone–containing cancer cells may lead to severe hyponatremia at the time of chemotherapy induction.
Other factors that may increase antidiuretic hormone secretion include nausea, pain, narcotics, and nicotine. Antineoplastic agents such as high-dose intravenous cyclophosphamide, vincristine, vinblastine, and cisplatin can also increase antidiuretic hormone secretion.
In cases of hyponatremia secondary to SIADH, urine osmolality is higher than plasma osmolality, and urine sodium is determined by sodium intake. In patients with SIADH, urine sodium is usually higher than 40 mEq/L. Fluid restriction (usually 500-1,500 mL of free water a day), an increase in salt intake, and occasionally, loop diuretics are attempted first in most cases of SIADH when the patient is asymptomatic or has mild symptoms. In the presence of severe symptoms (seizures or obtundation), hypertonic saline infusions might be needed with close and frequent monitoring of sodium levels to avoid rapid correction and possible osmotic demyelination syndrome (previously called central pontine myelinolysis). Demeclocycline (600-1,200 mg/d) can be used in cases in which hyponatremia does not respond to more fluid restriction. Vasopressin receptor (V2) antagonists (tolvaptan and conivaptan) have been approved by the US Food and Drug Administration for treatment of clinically significant hypervolemic or euvolemic hyponatremia associated with heart failure or SIADH (24).
Central diabetes insipidus can occur after surgery for brain tumors and occasionally in cases of tumors near the sella or the hypothalamus that invade the neurohypophysis or disrupt the pituitary stalk. These cases are often recognized by a clinical presentation of polyuria or polydipsia and are usually treated with 1-deamino-8-D-arginine vasopressin (subcutaneously, intranasally, or orally) to control the symptoms and correct the associated hypernatremia.
Nephrogenic diabetes insipidus can also occur in patients with cancer, and multiple antineoplastic agents have been described in association with this syndrome. Ifosfamide is well known to induce damage to the proximal renal tubule and, to a lesser extent, the distal renal tubule, and thereby induce nephrogenic diabetes insipidus. Streptozocin has also been reported to cause nephrogenic diabetes insipidus.
In addition to being associated with diabetes insipidus, hypernatremia in patients with cancer is commonly caused by insufficiency of free water, especially when patients are on parenteral or tube feeding regimens or are too debilitated to obtain water for themselves.
DISORDERS OF BONE AND BONE MINERAL METABOLISM
Normal bone remodeling requires a delicate balance between bone formation by osteoblasts and bone resorption by osteoclasts. Antineoplastic therapy may affect this balance by increasing the activity of osteoclasts (eg, interleukin-2) and sometimes by having direct toxic effects on osteoblast function. Hormones and cytokines (ie, parathyroid hormone [PTH], PTH-related peptide, and interleukin-1) can also affect the overall bone turnover rate.
Bone mineral loss is one of the side effects of cancer treatment. Hormone-suppressive therapies, chemotherapeutics, and corticosteroids can cause osteoporosis in cancer patients (25). Improved oncologic treatments and patient longevity have increased the importance of skeletal health in cancer patients.
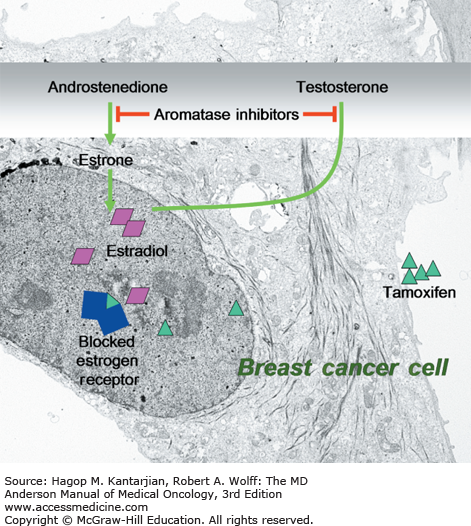
Breast cancer patients are at high risk for osteoporosis after hormone-suppressive therapy (26). Aromatase inhibitors, including anastrozole and letrozole, have been shown to decrease bone density and increase the rate of fractures in postmenopausal women. This is in sharp contrast to the positive effect on bone (both bone mineral density and fracture rates) seen with tamoxifen, a selective estrogen receptor modulator (Fig. 52-1). In the ATAC (Arimidex [anastrozole], Tamoxifen, Alone or in Combination) trial, 9,366 postmenopausal women with invasive operable breast cancer who had completed primary therapy were randomly assigned to receive anastrozole, tamoxifen, or both. More fractures were seen in patients receiving anastrozole compared with patients on tamoxifen (27). According to these findings, patients should undergo bone mineral density testing prior to treatment with aromatase inhibitors, and annual follow-up should be conducted thereafter. Antiresorptive therapy is usually added if a patient’s bone density is within the osteoporotic range (T score ≤ –2.5) before treatment or if a significant decline in bone mineral density was seen during follow-up.
FIGURE 52-1
Mechanism of action of selective estrogen receptor modulators and aromatase inhibitors. Aromatase inhibitors, including anastrozole and letrozole, inhibit conversion of androstenedion or testosterone to estrone, which causes a decrease in bone mineral density. Tamoxifene, a selective estrogen receptor modulator, has a positive effect on bone.
Osteoporosis can also occur in prostate cancer patients treated with androgen deprivation therapy (ADT), which can consist of gonadotropin-releasing hormone (GnRH) agonists, nonsteroidal antiandrogens, bilateral orchiectomy, and/or androgen blockers. These therapies can result in bone mineral loss and fractures (28) due to hypogonadism that increases bone resorption. The risk of fractures is higher in prostate cancer patients undergoing ADT than in those not undergoing ADT (29).
Patients with prostate cancer should be evaluated with dual-energy x-ray absorptiometry before starting ADT. Patients with fragility fractures or osteoporosis should be offered antiosteoporosis treatment. Before such treatment, nonpharmacologic approaches such as lifestyle modifications and calcium and vitamin D supplementation can be helpful (30).
Antineoplastic agents have also been implicated in chemotherapy-associated osteoporosis. Prolonged therapy with oral methotrexate for acute lymphoblastic leukemia has led to distal extremity pain, severe osteoporosis, and associated fractures, with significant improvement after cessation of methotrexate therapy (31). Other agents reported to reduce bone density include cisplatin and carboplatin. In addition, many chemotherapy protocols include corticosteroids, which are known to decrease bone density and increase the risk of fractures.
Patients who have undergone bone marrow transplantation have been reported to have low bone mass. The reduced bone density is likely to be secondary to the long-term side effects of bone marrow radiation, chemotherapy, corticosteroids, and hypogonadism.
Osteoblasts and osteoclasts are under the influence of many hormonal and signaling pathways, including tyrosine kinase receptors for platelet-derived growth factor (PDGF receptors α and β) and c-Abl. Activation of the PDGF pathway improves bone mineral density in ovariectomized rats and accelerates fracture healing. The absence of c-Abl is associated with impaired osteoblast maturation, leading to an osteoporosis phenotype (32).
Multikinase inhibitors such as sorafenib, sunitinib, and imatinib, among others, can inhibit pathways that affect bone remodeling. Not much is known about the clinical effects of tyrosine kinase inhibitors, small molecules with variable receptor affinity and an intracellular signal blocking effect, on bone. Imatinib has been the best studied and will be discussed here.
After 2 to 4 years of treatment with imatinib in patients with chronic myelogenous leukemia, a significant increase in the volume of the trabecular bone in the iliac crest has been observed (33). Pre-osteoblast cells exposed to imatinib undergo suppression of PDGF-induced PI3 kinase/Akt activation with upregulation of genes associated with osteoblast differentiation and bone formation.
More studies are needed to determine the effects of imatinib and other tyrosine kinase inhibitors on bone and bone mineral metabolism.
Osteomalacia occurs when normal mineralization of the organic bone matrix fails. In children, abnormal mineralization and maturation of the growth plate at the epiphysis is called rickets. Nutritional deficiency (especially vitamin D deficiency) and renal wasting of phosphorus leading to hypocalcemia or hypophosphatemia are among the common causes of osteomalacia. Other contributing factors include drugs such as anticonvulsants or aluminum and systemic acidosis. Antineoplastic agents can also cause or worsen osteomalacia.
Ifosfamide-induced tubular damage leads to renal phosphate wasting, hypophosphatemia, and rickets. Although renal and skeletal consequences of ifosfamide therapy have been well described in children, only four adult patients with osteomalacia have been reported (34). Another antineoplastic agent, the estrogen derivative estramustine, used in prostate cancer metastatic to bone, can cause hypocalcemia, hypophosphatemia, secondary hyperparathyroidism, and osteomalacia with normal vitamin D levels (35).
Calcium homeostasis is normally maintained by the interplay of PTH, calcitonin, phosphorus, and vitamin D metabolites in several target organs, including bones, parathyroid glands, intestines, and kidneys. In patients with cancer, multiple factors can affect this delicate balance, including nutritional status, medications, and tumor secretion of cytokines, hormones, or other humoral factors.
Hypercalcemia occurs in 5% to 10% of all patients with advanced cancer, and severe hypercalcemia (calcium level >12 mg/dL) is seen in about 0.5% of all patients with cancer (36). Squamous carcinoma, renal cell carcinoma, non–small cell lung carcinoma, breast carcinoma, leukemia, non-Hodgkin lymphoma, and multiple myeloma are among the most common malignancies associated with hypercalcemia. Retinoic acid derivatives have been reported to induce hypercalcemia in treatment of acute promyelocytic leukemia (37).
Hyperparathyroidism occurs 2.5 to 3 times more often in patients treated with low-dose (2-7.5 Gy) external radiation to the head and neck area than in the age-matched control population. Hyperparathyroidism after high-dose irradiation is uncommon. Radiation exposure from radioactive iodine treatment has also been reported in association with hyperparathyroidism (38).
Many factors can increase a cancer patient’s risk of hypocalcemia. These factors include the patient’s nutritional status, the antineoplastic agents used, and the type of surgical procedures performed (eg, neck dissection). Cytotoxic chemotherapy can result in tumor lysis syndrome and its resultant hypocalcemia, as commonly seen in treatment of hematologic malignancies. Hyperphosphatemia, hyperkalemia, hypocalcemia, and hyperuricemia can occur after induction chemotherapy; it is of vital importance to prevent the complications of tumor lysis by hydration, alkaline diuresis, inhibition of uric acid synthesis, and administration of oral calcium or aluminum-based compounds to bind intestinal phosphate and enhance calcium absorption. Intravenous calcium administration can potentially cause calcium phosphate precipitation in the presence of severe hyperphosphatemia and should be used with extreme caution. Dialysis may be needed in cases of symptomatic hypocalcemia and serum phosphorus levels higher than 10 mg/dL.
Cisplatin has been associated with hypocalcemia. One proposed mechanism of cisplatin’s ability to induce hypocalcemia is through hypomagnesemia resulting in decreased PTH secretion (39). Other theories include inhibition of 1,25-dihydroxy-vitamin D formation by hypomagnesemia or cisplatin inhibition of mitochondrial function in the proximal renal tubule. Other agents reported to induce hypocalcemia include dactinomycin, carboplatin, doxorubicin, and cytarabine. Hypocalcemia has been seen following administration of bisphosphonates (zoledronic acid and pamidronate) or denosumab (a monoclonal antibody that inhibits receptor activator of nuclear factor-κB ligand [RANKL]), both used to reduce skeletal complications in treatment and prevention of advanced malignancies involving the bone (40). Serum calcium levels and 25-hydroxyvitamin D levels should be checked prior to and during therapy with bisphosphonates or denosumab.
Hypomagnesemia is a well-known side effect in patients receiving platinum-based chemotherapy. Cisplatin has toxic effects on the kidneys, causing morphologic changes and necrosis in the proximal tubule, a major site of magnesium reabsorption. Hypomagnesemia is a frequent complication of cisplatin chemotherapy, affecting up to 90% of patients; 10% of these patients have symptoms of muscle weakness, tremulousness, and dizziness. A recent study showed that premedication with magnesium reduced cisplatin-induced nephrotoxicity in patients with thoracic cancers (41).
Carboplatin, a second-generation platinum compound, was developed to reduce the side effects of cisplatin. However, hypomagnesemia has been seen with increasing frequency and severity at higher doses of carboplatin.
Oxaliplatin, a third-generation platinum derivative that has become an integral part of various chemotherapy protocols, particularly in advanced colorectal cancer, has dose-limiting cumulative sensory neurotoxicity similar to that of cisplatin. Oxaliplatin chelates to calcium and decreases magnesium levels. Hypomagnesemia was seen in 11% of patients with advanced epithelial ovarian cancer treated with oxaliplatin in a phase II trial (42). Oxaliplatin is considered to carry a lower risk for hypomagnesemia compared with cisplatin and carboplatin.
Cetuximab, a monoclonal antibody against the epithelial growth factor receptor (EGFR), is used to treat metastatic colon cancer. Because EGFR is common in the loop of Henle, cetuximab blocks reabsorption of magnesium in the kidneys. Increased renal wasting of magnesium can cause supplement-resistant hypomagnesemia. Magnesium levels should be checked before and during cetuximab therapy. Cetuximab-induced hypomagnesemia is also used as a marker of worse overall survival (43).