Fig. 23.1
Novel immune agents targeting immune signaling. MDX-1106 is a monoclonal antibody with affinity for PD-1. Binding of PD-1 on the T-cell surface to PD-L1/2 on the antigen-presenting cell (APC) leads to induction of T-cell anergy. In contrast to MDX-1106, tremelimumab and ipilimumab bind to CTLA4, preventing its interaction with B7 and promoting T-cell proliferation
Notably, PD-1 and PD-L1 inhibition is the subject of another chapter in the current manuscript, and will therefore not be explored in detail here. These agents have strong potential to impact the therapeutic landscape of mRCC. As one example, the PD-1 inhibitor nivolumab has shown impressive clinical outcome in several recently reported monotherapy trials [31–33]. An ongoing phase III study will compare nivolumab to everolimus in patients with prior exposure to VEGF-directed therapies [34]. Compelling phase I data for the combination of nivolumab with another checkpoint inhibitor, ipilimumab, has led to a phase III front-line trial assessing this combination [35]. These pivotal trials have the potential to drastically change the current treatment paradigm.
23.2.3 Denileukin Diftitox
Several attempts have been made to build upon current immunotherapeutic regimens. The agent denileukin diftitox (DD), approved for the treatment of CD25-positive non-Hodgkin’s lymphoma, has been noted to decrease regulatory T-cell (Treg) activity [36]. Given this property, it was thought that DD therapy would augment the activity of IL-2, which has the generalized effect of increasing all T-cell populations (both effector T-cells and Tregs) [37]. A pilot study examined a total of 18 patients with mRCC; a group of 3 patients were initially evaluated for toxicity – the remainder were enrolled after no atypical toxicities were observed [38]. Grade 3/4 toxicities were observed in 11 patients (61 %) receiving high-dose IL-2 and DD, with the most common toxicities including capillary leak syndrome and atrial fibrillation. Of 15 evaluable patients, 5 patients (33 %) demonstrated a response, including three CRs. Peripheral blood analyses did, in fact, reveal substantial reductions in Tregs with DD therapy, declining 56 % from baseline. Further studies are needed to clarify both the efficacy and toxicity of this regimen.
23.2.4 Targeting IL-6
The rationale for targeting IL-6 is multifold; in the context of RCC, elevated levels have been associated with increased metastasis and poor clinical outcome [39]. In addition, increasing levels of IL-6 have been associated with decreasing responsive to therapies such as IL-2 [40]. Rossi et al. reported a phase I/II study of the anti-IL-6 monoclonal antibody, CNTO 328 [41]. Patients had documented mRCC with detectable C-reactive protein (CRP) levels. A total of 11 patients were enrolled in the dose-finding phase I component of the study, and an additional 37 patients were included in the phase II component of the study. In the phase II component, patients were randomized to three schedules of CNTO 328, either 3 mg/kg or 6 mg/kg every 3 weeks for 4 cycles (regimen 1) or every 2 weeks for a total of 6 cycles (regimen 2). The majority of patients enrolled had received prior therapy for mRCC. With respect to efficacy, 1 of 20 patients receiving regimen 1 achieved a PR, while 10 patients (50 %) exhibited SD as a best response. Of the 17 patients receiving regimen 2, no patients achieved an objective response, although 11 patients (65 %) had SD for a median of 80 days. The toxicity profile of CNTO 328 appeared favorable, with no DLTs in the phase I component of the study. There were several serious adverse events (SAEs) recorded, however – one patient receiving regimen 1 suffered from grade 4 cardiac failure after receiving three doses of CNTO 328. Four other SAEs were not ultimately attributed to the antibody. Given the low level of activity seen with CNTO328 in this experience, it is unclear whether further development of the agent is warranted. If pursued, the agent will need to be assessed in combination with other therapies (Table 23.1).
Table 23.1
Selected emerging immune therapies for mRCC
Agent | Description | Current status/summary of available data |
|---|---|---|
AGS-003 | Autologous dendritic cell vaccine | Phase II combination studies with sunitinib reported, with encouraging PFS seen in intermediate- and poor-risk patients. Phase III study underway |
IMA901 | Vaccine comprised of tumor-associated peptides | Phase II studies reported, with encouraging activity in those patients in whom an immune response is elicited. Phase III study completed; results pending |
TG4010 | Vaccinia virus expressing IL-2 and MUC-1 antigen | Phase II studies reported, with limited toxicity but no objective response |
Nivolumab (BMS-936558) | Monoclonal antibody directed at PD-1 | Phase I study included patients with mRCC with encouraging clinical benefit rate and modest toxicity. Phase III assessment underway |
Ipilimumab | Monoclonal antibody directed at CTLA4 | Phase II data shows higher response rates among patients who incurred immune-related adverse events (i.e., autoimmune hypophysitis, colitis, etc.) Phase III assessment in combination with nivolumab |
Tremelimumab | Monoclonal antibody directed at CTLA4 | Phase I study in combination with sunitinib therapy shows substantial toxicity |
Denileukin diftitox | Diphtheria toxin fragment fused to IL-2 | Pilot study in mRCC showed substantial toxicity, but an appreciable response rate (20 % of patients achieved a CR) |
CNTO328 | Monoclonal antibody directed at IL-6 | Phase I/II study showed no objective responses; several serious adverse events were noted |
23.3 Angiogenesis Inhibitors: Beyond Direct VEGFR Inhibition
23.3.1 Dual Inhibition of VEGF and MET
There is substantial biological rationale for targeting c-MET signaling in mRCC. Firstly, alterations in VHL have been associated with constitutive activation of c-MET in clear cell RCC [42]. Secondly, in the context of papillary RCC, mutations in the tyrosine kinase domain of c-MET are well documented [43]. A phase II trial has been reported which assesses foretinib, a dual inhibitor of c-MET and VEGFR2, in papillary RCC [44]. Patients were divided into two cohorts, receiving the agent at either 240 mg oral daily on days 1–5 of a 14-day cycle or 80 mg oral daily. With a total of 74 patients enrolled, a PFS of 9.3 months was observed with a response rate of 13.5 %. Although the study failed to meet its primary endpoint based on response rate, the PFS in this population compares favorably to that observed with VEGF-TKIs for papillary mRCC. Furthermore, the study provided an opportunity for several key correlatives. Most notably, a total of ten patients were identified with germline MET mutations. Among this cohort, a response rate of 50 % was seen. The study thus points to the potential role of biomarker-based application of this agent in future trials.
A second dual VEGFR2/c-MET inhibitor, cabozantinib, has been assessed in the context of a phase I drug-drug interaction study with rosiglitazone [45]. In contrast to the evaluation of GSK089, this study was limited to patients with clear cell histology. The 25 patients with mRCC enrolled in this experience were heavily pretreated. Most patients had received at least one VEGF-directed therapy (88 %), and over 40 % of patients had received three or more prior therapies. Median PFS in this experience was an impressive 12.9 months, and a response rate of 28 % was observed. These encouraging data have led to a phase III study comparing cabozantinib and everolimus in patients with prior VEGF-directed therapy [46]. The Alliance cooperative group also conducted a randomized phase II study comparing cabozantinib to sunitinib.
A third agent, ARQ 197, specifically antagonizes c-MET. In a phase II study in patients with microphthalmia transcription family (MiT)-associated tumors, three of four patients (75 %) had SD as a best response with ARQ 197 therapy [47]. The agent has been examined in a randomized parallel phase II study (SWOG 1107), in which patients with papillary mRCC are treated with either ARQ 197 monotherapy or ARQ 197 in combination with erlotinib. The study is currently closed for an interim analysis.
A second approach to targeting the c-MET signaling axis is depletion of the relevant ligand, hepatocyte growth factor (HGF). Higher levels of this ligand have been associated with a poor prognosis in patients with clear cell RCC [48]. Furthermore, HGF appears to drive tumor growth in those patients with papillary RCC bearing mutations in c-MET [49]. AMG 102 is a humanized monoclonal antibody with affinity for HGF. In a phase II clinical trial, 61 patients with mRCC were treated with AMG 102 at two dose levels, either at 10 mg/kg or 20 mg/kg intravenous every 2 weeks. Patients enrolled had received at least one prior therapy, and although the majority had clear cell disease, 7 patients (11.5 %) had papillary RCC. One PR was observed, and 26 additional patients (43 %) had SD as a best response. Approximately one-third of patients incurred grade 3/4 toxicity, including edema, fatigue, and anorexia. Given the toxicity profile in combination with limited antitumor activity, it is unclear whether further single-agent evaluation of AMG 102 is warranted in mRCC.
23.3.2 Inhibition of Tie-2/Ang-1/2 Signaling
Outside of directly inhibiting VEGF-signaling, other strategies are being devised to inhibit angiogenesis (Fig. 23.2). Recently, attention has been directed to signaling via Tie-2, a cell surface receptor which promotes pericyte recruitment and maintenance of blood vessel integrity [50]. Two critical ligands have opposing effects on Tie-2 – angiopoietin-1 (Ang-1) activates the receptor, while angiopoietin-2 (Ang-2) inhibits the moiety [50, 51]. Ang-2 is overexpressed in a majority of cancer patients and when present is associated with an aggressive tumor phenotype and poor survival. In the context of RCC, Ang-2 expression is significantly higher in tumor tissue compared to normal renal parenchyma, correlated positively with Tie-2 levels. Furthermore, Ang-2 may be a biomarker of response to antiangiogenic therapy. Bullock et al. compared serum samples derived from 34 patients with mRCC to samples derived from 8 patients with stage I RCC [52]. Ang-2 levels were higher in the former group (median, 3,870 pg/mL v 2,489 pg/mL; P = 0.02). Of the patients with metastatic disease, 26 were evaluated while on therapy with sunitinib. In this group, Ang-2 decreased in 23 patients (88 %). Furthermore, at the time of progression, Ang-2 levels increased in the majority of patients. These preliminary studies provide support for attempts at pharmacologic inhibition of Ang-2. To this end, CVX-060 represents a combination of two peptides with a high affinity for Ang-2. The compound is being evaluated in a phase Ib clinical trial in combination with sunitinib therapy [53]. The combination appears to be well tolerated, and the phase Ib study will serve as a lead-in to a randomized phase II effort comparing sunitinib alone to the combination [54].
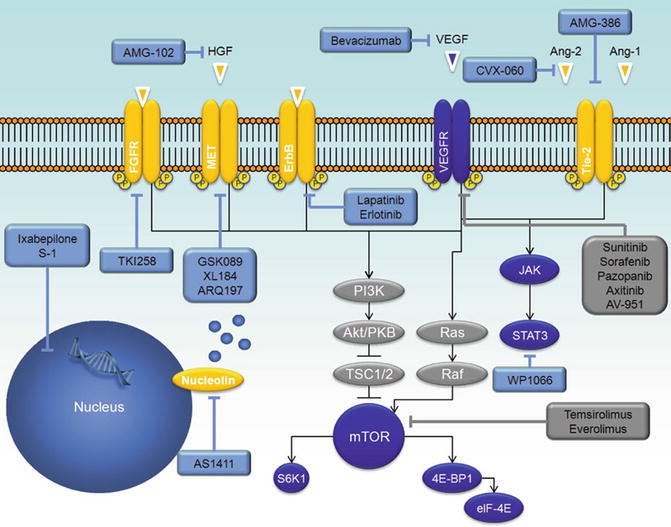

Fig. 23.2
Emerging agents for the treatment of mRCC. Approved agents are denoted in grey boxes, while agents currently in clinical development are denoted in blue boxes. Note that inhibitors of PI3K/Akt are delineated in other chapters in this textbook
While CVX-060 specifically targets Ang-2, there has been some suggestion that dual targeting of Ang-1 and Ang-2 may be a superior strategy [55]. AMG-386 is a peptibody that blocks the interaction of both Ang-1 and Ang-2 with Tie-2 [56]. Preclinical data suggests that VEGF-driven angiogenesis can be mitigated through increasing doses of AMG-386. The agent has been explored extensively in mRCC. A recent, randomized phase II study compared the combination of sorafenib (400 mg oral twice daily) with either one of two dose levels of AMG-386 (3 mg/kg IV weekly or 10 mg/kg weekly) or placebo [57]. Notably, patients who exhibited PD on the placebo arm were offered a continuation of sorafenib with the addition of AMG-386 at 10 mg/kg. The study included patients with clear cell mRCC who had received no prior systemic therapy. The primary endpoint of the study was progression-free survival (PFS).
Ultimately, no significant difference in PFS was observed among patients treated with AMG-386 at 3 mg/kg or 10 mg/kg (8.5 vs. 9.0 months, 95 %CI 0.68–1.14; P = 0.523) [57]. Furthermore, patients receiving placebo had a nearly identical PFS (9.0 months). The confirmed overall RR was higher for patients receiving low- and high-dose AMG-386 (37 % and 38 %, respectively) as compared to placebo (25 %). Toxicity on the experimental arms appeared to parallel that observed on the placebo arm, suggesting that AMG-386 was generally well tolerated and added little to the side effect profile of sorafenib. Although efficacy of AMG-386 was limited in this study, data from other malignancies suggest that doses in excess of 10 mg/kg may yield higher antitumor activity.
While the aforementioned agents specifically target the Ang/Tie signaling axis, regorafenib is an oral TKI that additional binds VEGF receptors and c-kit. This agent has the theoretical advantage of dual pathway inhibition of angiogenesis [58]. Phase I studies demonstrated activity for regorafenib in a number of tumor types including RCC, non-small cell lung cancer, and colorectal cancer with a recommended phase II dose of 160 mg per day for 21 days followed by a 7-day rest [59, 60]. On that basis, a phase II study of 49 evaluable patients given no prior systemic therapy for measurable clear cell predominant advanced or metastatic RCC was conducted [61]. The primary objective was to evaluate the antitumor activity and safety of regorafenib, while secondary objectives included the evaluation of pharmacokinetic and biomarker data [62]. The response rate was 31 % with an additional 50 % experiencing stable disease. Median progression-free survival was 8.2 months with the median overall survival not reached at the time of presentation. Grade 3 or 4 adverse events occurred in 33 (67 %) patients, most commonly hand-foot skin reaction (29 %), renal failure (10 %), and fatigue (8 %). Patients with higher baseline plasma levels of soluble Tie-1 were more likely to have major tumor shrinkage on therapy. Increased in plasma VEGF-A, VEGF-C, Ang-2, carbonic anhydrase 9, and CK18M30 (a marker of epithelial cell death) and decreased in VEGFR2, soluble Tie-1, and c-kit were seen on therapy. Increased CK18M30 and decreased c-kit were associated with response. Further data from this study are awaited. Regorafenib is being developed in colorectal and non-small cell lung cancer, but a decision on development in RCC is complex given crowding in that market with other VEGF-TKIs.
23.3.3 Thalidomide and Lenalidomide
While the precise mechanism of thalidomide and lenalidomide remains a matter of debate, the agents appear to have both antiangiogenic and immunomodulatory properties akin to other efficacious therapies for mRCC. There have been several attempts to characterize the activity of these agents in mRCC. Choueiri et al. have reported a phase II, open-label study including 28 patients who received lenalidomide at 25 mg oral daily for 3 weeks in a 4 week cycle [63]. Patients had received no more than 1 prior therapy and had a baseline ECOG PS of 0–1. Although no CRs were noted, three patients (11 %) demonstrated a PR and remained progression-free at a follow-up interval exceeding 15 months. Eleven patients (39 %) were noted to had SD >3 months. The median time to treatment failure was 3.7 months, and at the time of publication, median OS had not been reached. Fatigue, skin reactions, and hematologic toxicity constituted the most common grade 3/4 events. A slightly larger trial assessing lenalidomide included 40 patients with mRCC, again limiting entry to patients who had received no more than 1 prior therapy [64]. Among 39 evaluable patients, 4 patients (10 %) achieved an objective response (one CR and three PRs). An additional 20 patients (51 %) had SD lasting ≥6 months. Similarly to the previously noted experience, fatigue and hematologic toxicity constituted the most common adverse reactions. Both of these datasets emerged at roughly the time initial data was presented for the VEGF-TKIs. Although further development of single-agent lenalidomide for mRCC has not been aggressively pursued, there are currently efforts examining the combination of lenalidomide with other targeted agents for mRCC, including sunitinib and everolimus [65, 66].
Several therapeutic trials have also reported the clinical activity of thalidomide therapy in mRCC. Daliani et al. reported an experience including 20 patients with mRCC treated with thalidomide at a starting dose of 200 mg oral daily, with an upward titration to 1,200 mg oral daily as tolerated [67]. Patients had received a median of two prior therapies, primarily consisting of immunotherapy (HD IL-2 or IFN-α). Median TTP was 4.7 months, with a median survival of 18.1 months. Two patients (10.5 %) achieved a PR, and an additional nine patients (50 %) had SD in the range of 3–17 months. A larger experience reported by Escudier et al. assessed 40 patients with advanced disease, with a similar titration to 1,200 mg oral daily [68]. Two patients (5 %) experienced a PR, while nine patients (23 %) had SD lasting greater than 6 months. Significant toxicities were observed in this experience, with three patients experiencing a pulmonary embolism within 12 weeks of treatment initiation and one additional patient experiencing a venous thromboembolism. Neuropathy was observed in 100 % of patients who received thalidomide for a period of 12 months. Ultimately, although corroborating the marginal activity seen with thalidomide in mRCC, this larger experience suggested that the assessed dose could not be recommended due to the extent of toxicity.
Combinations of thalidomide with various agents have been explored. Desai et al. reported a phase II experience assessing the combination of gemcitabine and continuous infusion fluorouracil with thalidomide [69]. Ultimately, it was determined that thalidomide added little to the efficacy of the cytotoxic regimen but added substantial vascular toxicity. Combinations of thalidomide with immunotherapy have also been attempted; Hernberg et al. reported a phase II clinical trial evaluating the combination of IFN-α and thalidomide [70]. Although the regimen assessed appeared to be feasible, thalidomide added little to the anticipated clinical benefit from IFN-α alone. Thalidomide therapy has also been assessed in the adjuvant setting, with somewhat sobering results. Patients with high-grade T2 disease, T3/T4 disease, or nodal positivity were randomized to receive either thalidomide 300 mg oral daily for 24 months or observation. After enrollment of a total of 46 patients, there was an inferior 2-year recurrence-free survival (RFS) observed on the thalidomide arm (47.8 % v 69.3 %, P = 0.022).
23.3.4 Thrombospondin-1 Agonism
Activated by p53, thrombospondin-1 inhibits the activity of VEGF and basic fibroblast growth factor (bFGF), both putative mediators of angiogenesis [71, 72]. A phase II study examined two dose levels of the thrombospondin-1 analogue, ABT-510, in patients with treatment-naïve mRCC [73]. With a total of 103 patients enrolled, 51 patients were randomized to a dose of 10 mg subcutaneously twice daily, while 52 were randomized to receive 100 mg subcutaneously twice daily. The majority of patients in this study had clear cell disease (76 %) and had a baseline ECOG PS of 0 (70 %). There were no differences in PFS or RR between patients receiving 10 and 100 mg doses of ABT-510 (PFS: 4.2 vs. 3.3 months, respectively, P = 0.803; RR: 4 % v 0 %, respectively; P = 0.243). Although the agent had limited toxicity (a total of 4 grade 3/4 events were noted), the efficacy observed in this study was not thought to justify further investigation of the single agent.
23.4 Other Novel Targets in mRCC
23.4.1 Targeting Fibroblast Growth Factor Receptor (FGFR)
FGFR signaling is a putative escape mechanism for cancer cells exposed to VEGF-directed therapies [74]. Although the small-molecule dovitinib has affinity for the VEGF family of receptors and other receptor tyrosine kinases, it uniquely binds FGFR1-3 with high affinity [75]. A phase I/II study has explored the activity of dovitinib therapy in mRCC patients refractory to standard treatment [76]. The phase I component of the study was recently reported, including 20 patients that had received a range of prior therapies, including VEGF-TKIs (80 %), mTOR inhibitors (55 %), and the immunotherapy (15 %). Confirmed PRs were observed in two patients (10 %), and seven patients (35 %) achieved SD as a best response. Notably, in the subset of ten patients who had received both VEGF-TKIs and mTOR inhibitors, one patient exhibited a PR and six patients had SD as a best response.
Based on these encouraging preliminary results in a heavily refractory population, a phase III trial was performed to compare dovitinib to sorafenib as a third-line therapy in patients with mRCC that had received one VEGF-TKI and one mTOR inhibitor [77]. The study accrued a total of 570 patients and ultimately failed to meet its primary endpoint of improvement in PFS. PFS associated with dovitinib was 3.7 months, as compared to 3.6 months with sorafenib (P = 0.063). Although the study was negative, it does provide some insights into future benchmarks for clinical trials in mRCC done in the third-line setting.
23.4.2 ErbB Targeting
Several attempts have been made to assess the role of ErbB-directed therapies in mRCC. Preclinical studies in RCC-derived cell lines suggested that the presence of wild-type VHL was associated with increased responsiveness to the EGFR-directed monoclonal antibody C225 [78]. On the basis of these data, Southwest Oncology Group (SWOG) trial 0317 assessed the EGFR tyrosine kinase inhibitor erlotinib in patients with papillary renal cell carcinoma [79]. Patients in this study had not received prior chemotherapy or immunotherapy and were treated with erlotinib at 150 mg oral daily until the time of disease progression. Of 45 evaluable patients, 5 patients (11 %) achieved a response to therapy, with 24 additional patients achieving stable disease. The median OS in this population was 27 months. Although the study failed to meet the prespecified endpoints for response rate, the clinical benefit ascribed to erlotinib therapy was deemed to be encouraging. Several subsequent efforts have examined other combinations with erlotinib. Flaig et al. reported a study assessing erlotinib with sirolimus in patients with metastatic RCC (albeit not restricted to clear cell disease) [80]. Patients in this study had previously progressed on therapy with sunitinib or sorafenib therapy. No responses were observed to this regimen, and median PFS was 12 weeks. These data failed to support further exploration of this regimen as an alternative to other available second-line therapies. Combination therapy has also been assessed in the context of the treatment-naïve patient – a randomized phase II study comparing bevacizumab with or without erlotinib showed no difference in RR (14 % with the combination v 13 % with bevacizumab alone), and no benefit in PFS (9.9 months with the combination v 8.5 months with bevacizumab alone, P = 0.58) [81]. A separate regimen of bevacizumab, imatinib and erlotinib has also been explored in a phase I/II study; this regimen yielded unacceptable toxicity (grade 3/4 diarrhea, rash and fatigue) [82].
Outside of EGFR, other moieties in the ErbB family have been assessed as therapeutic targets in mRCC. As one notable example, a phase III clinical trial was conducted using the dual-targeting small-molecule inhibitor lapatinib, which antagonizes both EGFR and HER2. In this study, 416 patients with mRCC were randomized to receive either lapatinib or hormonal therapy (tamoxifen or medroxyprogesterone). Patients were eligible if any level of immunohistochemical staining for HER2 (1+, 2+ or 3+) was observed and if they had progressed on prior cytokine-based therapy. Median TTP was 15.3 weeks with lapatinib as compared to 15.4 weeks with hormonal therapy (P = 0.60). OS was also comparable between lapatinib and hormonal therapy (46.9 vs. 43.1 weeks, respectively; P = 0.29). In the subset of 241 patients with 3+ staining, there was a more appreciable difference in clinical outcome – there was a trend toward improvement in TTP with lapatinib therapy (15.1 vs. 10.9 weeks, P = 0.06) and a significant improvement in OS (46.0 vs. 37.9 weeks; P = 0.02).
23.4.3 Targeting Nucleolin
Oligonucleotide aptamers represent short nucleic acid sequences that exhibit conformal binding to proteins. The novel aptamer AS1411 represents one such molecule that specifically targets nucleolin. Nucleolin is a protein with multiple purported roles and is found predominantly in rapidly dividing cells [83]. It is presumed to function in ribosome production and chromatin organization in the nucleolus [84]. Further, it may serve as a cell surface receptor for a variety of ligand growth factors [85]. Preclinical data suggested antitumor activity of AS1411 in the DU145 prostate cancer cell line, stimulating further clinical development of this agent [86].
A phase II, single-arm trial was conducted to evaluate the efficacy of AS1411 in mRCC [87]. The agent was administered to patients with clear cell histology who had failed one or more prior therapies at a dose for 40 mg/kg/day for days 1–4 of a 28-day cycle. Patients received only 2 cycles of therapy. With 35 patients enrolled, 1 patient exhibited a PR, and 21 patients (60 %) had SD as a best response. No grade 4 or 5 toxicities were observed; the most common adverse effects were diarrhea and fatigue. It remains to be seen whether further combination studies of the drug will be pursued, given both the modest toxicity and efficacy of the agent (Table 23.2).
Table 23.2
Selected emerging agents for mRCC that inhibit novel angiogenic signaling axes
Agent | Description | Current status/summary of available data |
|---|---|---|
CVX-060 | Monoclonal antibody fused to 2 peptides with high affinity for Ang-2 | Phase Ib/II combination study with sunitinib ongoing |
AMG-386 | Peptibody that blocks the interaction of Ang-1/2 with Tie-2 | Phase II study comparing sorafenib with placebo or AMG-386 (at 2 dose levels) showed no improvement in PFS with the addition of AMG-386 |
Regorafenib | TKI with affinity for Tie-2, VEGFR2, and c-kit | Phase II study shows promising RR and PFS |
Thalidomide | Antiangiogenic and immunomodulatory agent | Phase II data for single-agent therapy shows modest clinical benefit with substantial toxicity. Combinations with immunotherapy and cytotoxic agents show little synergy but added toxicity. Adjuvant data from small series discouraging
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|