Ductal carcinoma in situ (DCIS) of the breast is a heterogeneous group of lesions with diverse malignant potential and a range of treatment options. It is the most rapidly growing subgroup among breast cancers, with more than 68,000 new cases diagnosed in the United States during 2008 (27% of all new cases of breast cancer).1 More than 90% are nonpalpable and discovered mammographically.
It is now well appreciated that DCIS is a stage in a neoplastic continuum in which most of the molecular changes that characterize invasive breast cancer are already present.2 Only quantitative changes in the expression of genes that have already been altered separate DCIS from invasive growth. Genes that may play a role in invasion control a number of functions, including angiogenesis, adhesion, cell motility, the composition of extracellular-matrix, and more. To date, genes that are uniquely associated with invasion have not been identified. DCIS is clearly the precursor lesion for most invasive ductal carcinomas, but not all DCIS lesions have sufficient time or the genetic ability to progress to invasive disease.3-5
Therapy for DCIS ranges from simple excision to various forms of wider excision (segmental resection, quadrant resection, oncoplastic resection, etc.), all of which may or may not be followed by radiation therapy. When breast preservation is not feasible, total mastectomy, with or without immediate reconstruction, is generally performed.
Since DCIS is a heterogeneous group of lesions rather than a single entity,6,7 and because patients have a wide range of personal needs that must be considered during treatment selection, it is clear that no single approach will be appropriate for all forms of the disease or for all patients. At the current time, treatment decisions are based upon a variety of measurable parameters (tumor extent, margin width, nuclear grade, the presence or absence of comedonecrosis, age, etc), as well as physician experience and bias, and upon randomized trial data, which suggest that all conservatively treated patients should be managed with postexcisional radiation therapy.
There have been dramatic changes in the frequency, clinical importance, and treatment of DCIS in the past 30 years. Before mammography was common, DCIS was rare, representing less than 1% of all breast cancer.8 Today, DCIS is common, representing 27% of all newly diagnosed cases and as many as 30% to 50% of cases of breast cancer diagnosed by mammography.1,9-13
Until approximately 20 years ago, the treatment for most patients with DCIS was mastectomy. Today, almost 75% of newly diagnosed patients with DCIS are treated with breast preservation.16 In the past, when mastectomy was common, reconstruction was uncommon; if it was performed, it was generally done as a delayed procedure. Today, reconstruction for patients with DCIS treated by mastectomy is common; when it is performed, it is generally done immediately, at the time of mastectomy. In the past, when a mastectomy was performed, large amounts of skin were discarded. Today, it is considered perfectly safe to perform a skin-sparing mastectomy for DCIS and in some instances, nipple–areola-sparing mastectomy. In the past, there was little confusion. All breast cancers including DCIS were considered essentially the same and mastectomy was the only treatment. Today, we recognize that all breast cancers are different and there is a range of acceptable treatments for every lesion. For those who chose breast conservation, there continues to be a debate as to whether radiation therapy is necessary in every case. These changes were brought about by a number of factors. Most important were increased mammographic utilization and the acceptance of breast-conservation therapy for invasive breast cancer.
The widespread use of mammography changed the way DCIS was detected. In addition, it changed the nature of the disease detected by allowing us to enter the neoplastic continuum at an earlier time. It is interesting to note the impact that mammography had on the number of DCIS cases diagnosed and the way they were diagnosed at the Breast Center in Van Nuys, California.17
From 1979 to 1981, the Van Nuys Group treated a total of only 15 patients with DCIS, 5 per year. Only 2 lesions (13%) were nonpalpable and detected by mammography. In other words, 13 patients (87%) presented with clinically apparent disease. Two state-of-the-art mammography units and a full-time experienced radiologist were added in 1982, and the number of new DCIS cases increased to more than 30 per year, most of them nonpalpable. When a third machine was added in 1987, the number of new cases increased to 40 new cases per year. In 1994, the Van Nuys Group added a fourth mammography machine and a prone stereotactic biopsy unit. Analysis of the entire series of 1363 patients through April 2008 shows that 1201 lesions (88%) were nonpalpable (subclinical). If we look at only those diagnosed during the last 5 years at the USC/Norris Cancer Center, 95% were nonpalpable.
The second factor that changed how we think about DCIS was the acceptance of breast conservation therapy (lumpectomy, axillary node dissection, and radiation therapy) for patients with invasive breast cancer. Until 1981, the treatment for most patients with any form of breast cancer was generally mastectomy. Since that time, numerous prospective randomized trials have shown an equivalent rate of survival for selected patients with invasive breast cancer treated with breast conservation therapy.18-23 Based on these results, it made little sense to continue treating less aggressive DCIS with mastectomy while treating more aggressive invasive breast cancer with breast preservation.
In 1979, Azzopardi and associates aptly noted that the various architectural classifications of DCIS had no apparent significance for clinical outcome.24 However, at that time, all DCIS was treated by total mastectomy, and therefore there was no opportunity to evaluate the impact of architectural classification on local recurrence. Grading of DCIS can be thought of as classification independent of architectural pattern. Grading was introduced for DCIS in 1989 to determine whether it had significant impact on local recurrence in conservatively treated patients. It was based on the nuclear grading component of the Scarf–Bloom–Richardson system.25 Necrosis was included in the grading but was not used initially to distinguish subsets. Lagios and associates were able to show significant differences in local recurrence rates with 3 grades based on the nuclear grade and presence of necrosis largely because 2 other significant prognostic variables were controlled in their study population. Tumor size (extent) was required to be 25 mm or less, and margins had to be adequate: minimally 1 mm but most were larger. Solin and colleagues, in a dichotomous classification (high grade = nuclear grade 3 with necrosis versus everything else), similarly showed significant differences at 5 years.26
The division by architecture alone, comedo versus noncomedo, is an oversimplification and does not work if the purpose of the division is to sort the patients into those with a high risk of local recurrence versus those with a low risk. It is not uncommon for high nuclear grade noncomedo lesions to express markers similar to those of high-grade comedo lesions and to have a risk of local recurrence similar to comedo lesions. Adding to the confusion is the fact that mixtures of various architectural subtypes within a single biopsy specimen are common. In my personal series, more than 70% of all lesions had significant amounts of 2 or more architectural subtypes, making division into a predominant architectural subtype problematic.
Numerous subsequent classifications, based on nuclear grade and necrosis, have shown a similar association of grade and local recurrence rate in conservatively treated patents. Holland and associates used surrogate nomenclature for high, intermediate, and low grades but accomplished the same thing.27 The Van Nuys classification, which is based on nuclear grade and necrosis, has been shown to be the most reproducible in actual practice.28,29 In the Van Nuys classification, necrosis is used in absolute terms: any zonal necrosis no matter how limited classifies the patient as exhibiting necrosis—as opposed to the NSABP-B17 randomized trial, in which a third of the ducts must exhibit necrosis to qualify as high grade. This leads to the anomalous situation that a DCIS with nuclear grade 3 but only 20% of ducts exhibiting zonal necrosis would be classified in the low-risk comparison group. DCIS with nuclear grade 1 or 2 is classified on the basis of zonal necrosis into that without necrosis (class 1 = low grade) and that with necrosis (class 2 = intermediate grade).30 The biggest impact of grade is seen in circumstances where the other variables are controlled.13,25 In corollary fashion in circumstances where the prognostic variables cannot be defined (B17, B24, EORTC 10853, and Swed DCIS trials), grade has a more limited impact.
In 1995, the Van Nuys Group introduced a new pathologic DCIS classification31 based on the presence or absence of high nuclear grade and comedo-type necrosis (the Van Nuys Classification). The Van Nuys Group chose high nuclear grade as the most important factor in their classification because there was general agreement that patients with high nuclear grade lesions were more likely to recur at a higher rate and in a shorter time period after breast conservation than patients with low nuclear grade lesions.25,26,32-35 Comedo-type necrosis was chosen because its presence also suggests a poorer prognosis36,37 and it is easy to recognize.38
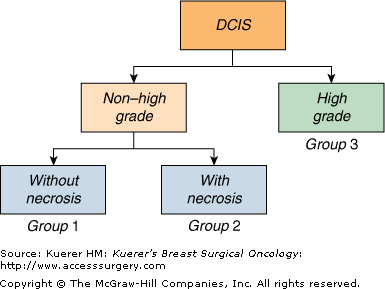
The pathologist, using standardized criteria, as noted in the next paragraph first determines whether the lesion is high nuclear grade (nuclear grade 3) or non–high nuclear grade (nuclear grades 1 or 2). Then, the presence or absence of necrosis is assessed in the non–high-grade lesions. This results in 3 groups (Fig. 18-1).
Nuclear grade is scored by previously described methods.31 Essentially, low-grade nuclei (grade 1) are defined as nuclei 1 to 1.5 red blood cells in diameter with diffuse chromatin and unapparent nucleoli. Intermediate nuclei (grade 2) are defined as nuclei 1.5 to 2 red blood cells in diameter with coarse chromatin and infrequent nucleoli. High-grade nuclei (grade 3) are defined as nuclei with a diameter greater than 2 red blood cells, with vesicular chromatin, and one or more nucleoli.
In the Van Nuys classification, no requirement is made for a minimum or specific amount of high nuclear grade DCIS, nor is any requirement made for a minimum amount of comedo-type necrosis. Occasional desquamated or individually necrotic cells are ignored and are not scored as comedo-type necrosis.
The most difficult part of most classifications is nuclear grading, particularly the intermediate-grade lesions. The subtleties of the intermediate grade lesion are not important to the Van Nuys classification; only nuclear grade 3 need be recognized. The cells must be large and pleomorphic, lack architectural differentiation and polarity, have prominent nucleoli and coarse clumped chromatin, and generally show mitoses.31,36
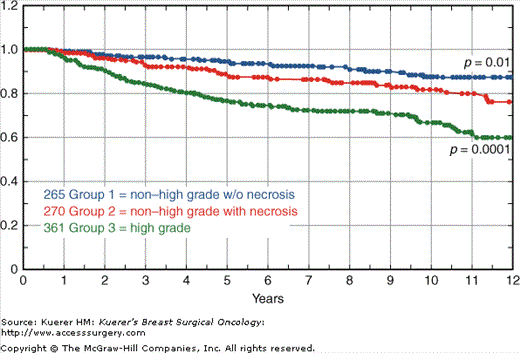
The Van Nuys classification is useful because it divides DCIS into 3 different biologic groups with different risks of local recurrence after breast conservation therapy (Fig. 18-2). This pathologic classification, when combined with tumor size, age, and margin status, is an integral part of the USC/Van Nuys Prognostic Index (USC/VNPI), a system that will be discussed in detail.
Which DCIS lesions will become invasive and when will that happen? These are the most important questions in the DCIS field today. Currently, there is intense study of molecular and genetic factors associated with the progression of normal breast epithelium through hyperplastic and atypical hyperplastic changes to DCIS and then to invasive breast cancer. Most of the genetic and epigenetic changes present in invasive breast cancer are already present in DCIS. To date, no genes uniquely associated with invasive cancer have been identified.2,16 As DCIS progresses to invasive breast cancer, quantitative changes in the expression of genes related to angiogenesis, adhesion, cell motility, and the composition of the extracellular matrix may occur.2,16 Using gene-array technology, researchers are attempting to identify high-risk patterns that will require quicker and more aggressive treatment.
The retrospective studies of Page and associates39,40 and Rosen and associates41 provide some evidence of the natural history of untreated DCIS. In these studies, patients with noncomedo DCIS were initially misdiagnosed as having benign lesions and therefore went untreated. Subsequently, approximately 25% to 35% of these patients developed invasive breast cancer, generally within 10 years.39,40 Had the lesions been high-grade comedo DCIS, the invasive breast cancer rate likely would have been higher than 35% and the time to invasive recurrence shorter. With few exceptions, in both of these studies, the invasive breast carcinoma was of the ductal type and located at the site of the original DCIS. These findings suggest that not all DCIS lesions progress to invasive breast cancer or become clinically significant within the patient’s lifetime.42,43
Page and associates recently updated their series.39,40,44 Of 28 women with low-grade DCIS misdiagnosed as benign and treated with biopsy between 1950 and 1968, 11 patients have recurred locally with invasive breast cancer (39%). Eight patients developed recurrence within the first 12 years (28%). The remaining 3 were diagnosed over 23 to 42 years. Five patients developed metastatic breast cancer (18%) and died from the disease within 7 years of developing invasive breast cancer. These recurrences and mortality rates, at first glance, seem alarmingly high. However, they are only slightly worse than what can be expected with long-term follow-up of patients with lobular carcinoma in situ, a disease that most clinicians are willing to treat with careful clinical follow-up.45 In addition, these patients were treated with biopsy only. No attempt was made to excise these lesions with a clear surgical margin. The natural history of low-grade DCIS can extend over 40 years and is markedly different from that of high-grade DCIS.
The incidence of microinvasion is difficult to quantitate because until recently there was no formal and universally accepted definition of exactly what constitutes microinvasion. The 1997 edition of The Manual for Cancer Staging (fifth edition) carried the first official definition of what is now classified as pT1mic, and reads as follows:
Microinvasion is the extension of cancer cells beyond the basement membrane into adjacent tissues with no focus more than 0.1 cm in greatest dimension. When there are multiple foci of microinvasion the size of only the largest focus is used to classify the microinvasion (do not use the sum of all individual foci). The presence of multiple foci of microinvasion should be noted, as it is with multiple larger invasive carcinomas.
The reported incidence of occult invasion (invasive disease at mastectomy in patients with a biopsy diagnosis of DCIS) varies greatly, ranging from as little as 2% to as much as 21%.46 This problem was addressed in the investigations of Lagios and associates.25,34
Lagios and colleagues performed a meticulous serial subgross examination correlated with specimen radiography. Occult invasion was found in 13 of 111 mastectomy specimens from patients who had initially undergone excisional biopsy of DCIS. All occult invasive cancers were associated with DCIS greater than 45 mm in extent; the incidence of occult invasion approached 50% for DCIS greater than 55 mm. In the study of Gump and associates,47 foci of occult invasion were found in 11% of patients with palpable DCIS but in no patients with clinically occult DCIS. These results suggest a correlation between the size of the DCIS lesion and the incidence of occult invasion. The studies of de Mascarel and coworkers have also shown an association of greater disease extent and microinvasion.48 Clearly, as the size of the DCIS lesion increases, microinvasion and occult invasion become more likely.
If even the smallest amount of invasion is found, the lesion should not be classified as DCIS. It is a T1mic (if the largest invasive component is 1 mm or less) with an extensive intraductal component (EIC). If the invasive component is 1.1 to 5 mm, it is a T1a lesion with EIC. If there is only a single focus of invasion, these patients do quite well. When there are many tiny foci of invasion, these patients have a poorer prognosis than expected.10 In the de Mascarrel and associates study, microinvasion defined as single cells alone had no adverse impact on outcome; only those comprising small cohesive nests of cells did.48 The latter had a small but significantly worse outcome as compared to either DCIS or microinvasion defined as single cells (91% vs 98%).
Multicentricity is generally defined as DCIS in a quadrant other than the quadrant in which the original DCIS (index quadrant) was diagnosed. There must be normal breast tissue separating the 2 foci. However, definitions of multicentricity vary among investigators. Hence the reported incidence of multicentricity also varies. Rates from 0% to 78%,7,34,41,49-51 averaging about 30%, have been reported. Twenty years ago, the 30% average rate of multicentricity was used by surgeons as the rationale for mastectomy in patients with DCIS.
Holland and colleagues52 evaluated 82 mastectomy specimens by taking a whole-organ section every 5 mm. Each section was radiographed. Paraffin blocks were made from every radiographically suspicious focus. In addition, an average of 25 blocks was taken from the quadrant containing the index cancer; random samples were taken from all other quadrants, the central subareolar area, and the nipple. The microscopic extension of each lesion was verified on the radiographs. This technique permitted a 3-dimensional reconstruction of each lesion. This study demonstrated that most DCIS lesions involved more than 1 quadrant by continuous extension (23%), but most importantly, were unicentric (98.8%). Only 1 of 82 mastectomy specimens (1.2%) had “true” multicentric distribution with a separate lesion in a different quadrant. This study suggests that complete excision of a DCIS lesion is possible due to unicentricity but may be extremely difficult due to larger than expected size. In a recent update, Holland reported whole-organ studies in 119 patients, 118 of whom had unicentric disease.53 This information, when combined with the fact that most local recurrences are at or near the original DCIS, suggests that the problem of multicentricity per se is not important in the DCIS treatment decision-making process.
Multifocality is defined as separate foci of DCIS within the same ductal system. The studies of Holland and associates52,53 and Noguchi and coworkers54 suggest that a great deal of multifocality may be artifactual, resulting from looking at a 3-dimensional arborizing entity in 2 dimensions on a glass slide. It would be analogous to saying that the branches of a tree were not connected if the branches were cut at one plane, placed separately on a slide, and viewed in cross-section.39 Multifocality may be due to small gaps of DCIS or skip areas within ducts, as described by Faverly and colleagues.35
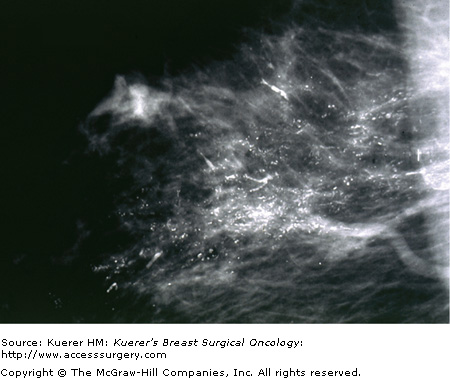
The importance of quality mammography cannot be overemphasized. Currently, most patients with DCIS (more than 90%) present with nonpalpable lesions. A few percent are detected as random findings during a biopsy for a breast thickening or some other benign fibrocystic change; most lesions, however, are detected by mammography. The most common mammographic findings are microcalcifications, frequently clustered, and generally without an associated soft-tissue abnormality. More than 80% of DCIS patients exhibit microcalcifications on preoperative mammography. The patterns of these microcalcifications may be focal, diffuse, or ductal, with variable size and shape. Patients with comedo DCIS tend to have “casting calcifications.” These are linear, branching, and bizarre and are almost pathognomonic for comedo DCIS (Fig. 18-3)55; however, nearly half of high-grade DCIS with comedo necrosis exhibits no microcalcifications
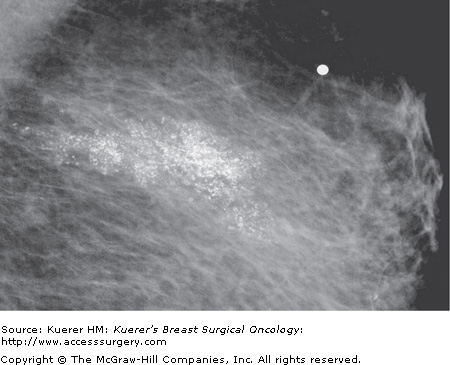
Thirty-two percent of noncomedo lesions in our personal series did not have mammographic calcifications, making them more difficult to find and the patients more difficult to follow, if treated conservatively. When noncomedo lesions are calcified, they tend to have fine psammomatous or indeterminate microcalcifications. Tabar has described these as granular powdery or crushed stone-like microcalcifications (Fig. 18-4).
A major problem confronting surgeons relates to the fact that calcifications do not always map out the entire DCIS lesion, particularly those of the noncomedo type. Even though all the calcifications are removed, in some cases, noncalcified DCIS may be left behind. Conversely, in some patients, the majority of the calcifications are benign and the calcifications “map out” a lesion bigger than the true DCIS lesion. In other words, the DCIS lesion may be smaller, larger, or the same size as the calcifications that lead to its identification. Calcifications more accurately approximate the size of high-grade comedo lesions than low-grade noncomedo lesions.52
Before mammography was common or of good quality, most DCIS was usually clinically apparent, diagnosed by palpation or inspection; it was gross disease. Gump and associates47 divided DCIS by method of diagnosis into gross and microscopic disease. Similarly, Schwartz and coworkers56 divided DCIS into 2 groups, clinical and subclinical. Both research groups thought patients presenting with a palpable mass, nipple discharge, or Paget disease of the nipple required more aggressive treatment. Schwartz and associates believed that palpable DCIS should be treated as though it were an invasive lesion. They suggested that the pathologist simply has not found the area of invasion. Although it makes perfect sense to believe that the change from nonpalpable to palpable disease is a poor prognostic sign, our group has not been able to demonstrate this for DCIS. In our series, when equivalent patients (by size and nuclear grade) with palpable and nonpalpable DCIS were compared, they did not differ in the rate of local recurrence or mortality. Lagios and coworkers had shown that patients with extensive disease (larger than 50 mm) generally present with palpable masses, nipple discharge, and so forth, and have a high frequency of microinvasion.25,34 Complete tissue processing is required to exclude invasion, and historically this has not been a standard practice.
If a patient’s mammogram shows an abnormality, most likely it will be microcalcifications, but it could be a nonpalpable mass or a subtle architectural distortion. At this point, additional radiologic workup needs to be performed. This may include compression mammography, magnification views, or ultrasonography. Magnetic resonance imaging (MRI) has become increasingly popular to map out the size and shape of biopsy-proven DCIS lesions or invasive breast cancers. I obtain a preoperative MRI on every patient with a diagnosis of breast cancer.
If radiologic workup shows an occult lesion that requires biopsy, there are multiple approaches: fine-needle aspiration biopsy (FNAB), core biopsy (with various types and sizes of needles), and directed surgical biopsy using guidewires or radioactive guidance. FNA is generally of little help for DCIS. With FNA, it is possible to obtain cancer cells, but because there is insufficient tissue, there is no architecture. So although the cytopathologist can say that malignant cells are present, the cytopathologist generally cannot say whether the lesion is invasive. Moreover, for DCIS of lower grades it may be impossible to distinguish ADH and low-grade invasion by cytology.
Stereotactic core biopsy became available in the early 1990s, and it is now widely used. Dedicated digital tables make this a precise tool in experienced hands. Currently large-gauge (11 gauge or larger) vacuum-assisted needles are the tools of choice for diagnosing DCIS. Ultrasound-guided biopsy also became very popular in the 1990s but is of less value for DCIS, since most DCIS lesions do not present with a mass that can be visualized by ultrasound. All suspicious microcalcifications, however, should be evaluated by ultrasound, since an invasive mass will be found in 5% to 15%.9
Open surgical biopsy should only be used if the lesion cannot be biopsied using minimally invasive techniques. This should be a rare event with current image-guided biopsy techniques.9 Needle-localized segmental resection should be a critical part of the treatment, not the diagnosis.
Whenever needle localization excision is performed, whether for diagnosis or treatment, intraoperative specimen radiography and correlation with the preoperative mammogram should be performed. Margins should be inked or dyed and specimens should be serially sectioned at 3- to 4-mm intervals. The tissue sections should be arranged and processed in sequence. Pathologic reporting should include determination of nuclear grade, an assessment of the presence or absence of necrosis, the measured size or extent of the lesion, and the margin status with measurement of the closest margin.57
Tumor size should be determined by direct measurement or ocular micrometry from stained slides for smaller lesions. For larger lesions, a combination of direct measurement and calculation, based on the distribution of the lesion in a sequential series of slides, should be used. The proximity of DCIS to an inked margin should be determined by direct measurement by ocular micrometry. The closest single distance between any involved duct containing DCIS and an inked margin should be reported.
If the lesion is large and the diagnosis unproven, either stereotactic or ultrasound-guided vacuum-assisted biopsy should be the first step. If the patient is motivated for breast conservation, a multiple-wire–directed oncoplastic excision can be planned. This will give the patient her best chance at 2 opposing goals: clear margins and good cosmesis. The best chance at completely removing a large lesion is with a large initial excision. The best chance at good cosmesis is with a small initial excision. It is the surgeon’s job to optimize these opposing goals. A large quadrant resection should never be performed as a diagnostic procedure. Such resections may result in significant deformity and are unwarranted without a preoperative tissue diagnosis.
Removal of nonpalpable lesions requires an integrated team of surgeon, radiologist, and pathologist. The radiologist who places the wires and interprets the specimen radiograph must be experienced, as must the surgeon who removes the lesion, and the pathologist who processes the tissue.
It is never easy to tell a patient that she has breast cancer. But is DCIS really cancer? From a biologic point of view, DCIS is unequivocally cancer. But when we think of cancer, we generally think of a disease that, if untreated, runs an inexorable course toward death. That is certainly not the case with DCIS.40 We must emphasize to the patient that she has a preinvasive cancerous lesion, which at this time is not a threat to her life. In our series of 1396 patients with DCIS, the raw mortality rate was 0.5%. Numerous other DCIS series60-65 confirm an extremely low mortality rate.
Patients often ask why there is any mortality rate at all, if DCIS is truly a noninvasive lesion. If DCIS recurs as an invasive lesion and the patient goes on to die from metastatic breast cancer, the source of the metastases is clear. But what about the patient who undergoes mastectomy and sometime later develops metastatic disease, or a patient who is treated with breast preservation who never develops a local invasive recurrence but still dies of metastatic breast cancer? These latter patients probably had an invasive focus with established metastases at the time of their original treatment but the invasive focus was missed during routine histopathologic evaluation. The ability to detect a small invasive lesion in a large resection is dependent on the thoroughness of the pathologic examination. It is an unfortunate truth that thorough examination of large resections and mastectomies is very uncommon in current practices as it was in the randomized trials.
One of the most frequent concerns expressed by patients once a diagnosis of cancer has been made is the fear that the cancer has spread. We are able to assure patients with DCIS that if no invasion was seen microscopically, the likelihood of systemic spread is essentially zero.
The patient needs to be educated that the term “breast cancer” encompasses a multitude of lesions of varying degrees of aggressiveness and lethal potential. The patient with DCIS needs to be reassured that she has a minimal lesion and that she is likely going to need some additional treatment, which may include surgery, radiation therapy, an antiestrogen, or some combination. She needs reassurance that she will not need chemotherapy, that her hair will not fall out, and that it is highly unlikely that she will die from this lesion. She will, of course, need careful clinical follow-up.
When evaluating the results of treatment for patients with breast cancer, a variety of end points must be considered. Important end points include local recurrence (both invasive and DCIS), regional recurrence (such as the axilla), distant recurrence, breast cancer–specific survival, overall survival, and quality of life. The importance of each end point varies depending on whether the patient has DCIS or invasive breast cancer
When treating invasive cancer, the most important end points are distant recurrence and breast cancer–specific survival; in other words, living with or dying from breast cancer. For invasive breast cancer, a variety of different systemic treatments have been shown to significantly improve survival. These include a wide range of chemotherapeutic regimens and endocrine treatments. Variations in local treatment were incorrectly thought not to affect survival.23,66 They do, however, affect local recurrence. Recently literature has suggested that for every 4 local invasive recurrences prevented, 1 breast cancer death is prevented.67
DCIS is similar to invasive breast cancer in that variations in local treatment affect local recurrence, but no study to date has shown a significant difference in distant disease-free or breast cancer–specific survival, regardless of any treatment (systemic or local), and no study is likely to show a difference since there are so few breast cancer deaths in patients with pure DCIS. The most important outcome measure, breast cancer–specific survival, is essentially the same no matter what local or systemic treatment is given. Consequently, local recurrence has become the most commonly used end point when evaluating treatment for patients with DCIS.
A meta-analysis of 4 randomized DCIS trials comparing excision plus radiation therapy versus excision alone was published in 2007. It contained 3665 patients. Radiation therapy increased local control by a statistically significant 60%, but overall survival was slightly worse in the radiotherapy group, with a relative risk of 1.08.64 These data are dissimilar to those of the Early Breast Cancer Trialists’ Collaborative Group and deserve further analysis.67 Half of the recurrences in the meta-analysis were DCIS and could not possibly affect survival. Of the remaining invasive recurrences, 80% to 90% were cured by early detection and treatment. This should result in a slight trend toward a lower survival for the excision alone group but exactly the opposite was seen, a nonsignificant trend toward a better survival. The authors of the meta-analysis feel that with longer follow-up, the higher local recurrence rate for excision alone will likely result in a lower overall survival at some point in time. But for the time being, that has not happened, and a detrimental effect secondary to radiation therapy must be considered a possibility.
Local recurrences are clearly important to prevent in patients treated with DCIS. They are demoralizing. They often lead to mastectomy, and theoretically, if they are invasive, they upstage the patient and are a threat to life. But protecting DCIS patients from local recurrence must be balanced against the potential detrimental effects of the treatments given.
Following breast-conserving treatment for DCIS, 40% to 50% of all local recurrences are invasive. About 10% to 20% of DCIS patients who develop local invasive recurrences develop distant metastases and die from breast cancer.68,69 In the long term, this could translate into a mortality rate of about 0% to 0.5% for patients treated with mastectomy, 1% to 2% for conservatively treated patients who receive radiation therapy (if there is no mortality associated with radiation therapy), and 2% to 3% for patients treated with excision alone. In order to save their breasts, many patients are willing to accept this theoretical, and presently statistically unproven potential risk associated with breast conservation therapy.