Fig. 11.1
Radiographic microcalcifications from DCIS on mammography in a patient with a prior implant
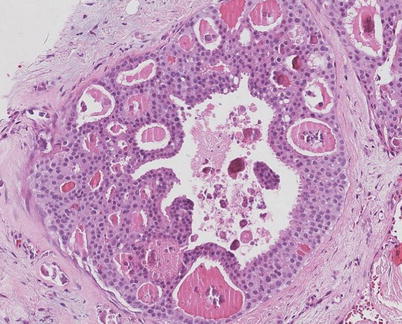
Fig. 11.2
Microcalcifications (irregular purple staining) in secretions (pink staining) in low grade DCIS
11.4.2 Ultrasound
Some academic centres routinely employ ultrasound on all women with an abnormal mammogram or a palpable mass. In the USA, the National Comprehensive Cancer Network (NCCN) guidelines [15] recommend that ultrasound evaluation is an optional part of the work-up for patients with early-stage breast disease. By contrast, the UK National Institute for Health and Care Excellence (NICE) guidelines [16] recommend ultrasound evaluation of the axilla as well as the breast for all patients with early invasive disease, but not per se for DCIS. Although not specifically designed to detect microcalcifications (unlike mammography), approximately 5–15% of patients with microcalcification will have a mass detectable on ultrasound.
11.4.3 MRI
A recent meta-analysis conducted to examine the effects of MRI on the surgical treatment of DCIS concluded that MRI in women with DCIS is not associated with an improvement in surgical outcomes [17]. In this analysis, there was no difference in the proportion of women with positive margins following breast conserving surgery (BCS) or in the reoperation rate for positive margins between patients having MRI and no MRI. Conversely, MRI was found to increase the odds of having mastectomy rather than an initial conservation approach. MRI use in women with DCIS does not appear to confer an oncological advantage [18] and has a lower sensitivity for DCIS compared with invasive cancer. The UK NICE guidelines caution against routine use of MRI in patients with DCIS and guides clinical teams to use this modality if there is a discrepancy regarding the extent of disease between clinical examination and other imaging modalities or if breast density precludes accurate mammographic assessment.
11.4.4 Biopsy
Any radiographically suspicious abnormality warrants biopsy. One option is fine needle aspiration (FNA) cytology. FNA offers the convenience of being a simple procedure with standard equipment (syringe and needle) for palpable lesions; however, cytological examination cannot distinguish in situ from invasive disease, and definitive preoperative diagnosis rates for screen-detected DCIS are poor (73% sensitivity compared with 94% sensitivity with core biopsy) [19]. At least partly for this reason, FNA alone is not recommended for screen-detected abnormalities in the UK National Health Service Breast Screening Programme (NHS BSP).
Core needle biopsy provides a more accurate assessment because the tissue architecture is retained and definitive diagnosis of DCIS, i.e. compared to invasive disease, can be made (with the proviso that only a small portion of the lesion is sampled and a small invasive focus may have been missed). Additionally, core needle biopsy may provide sufficient tissue for examination of hormone receptor status, which can have important implications for treatment. A core needle biopsy can be done as a stereotactic procedure for calcifications seen on mammogram or with ultrasound guidance in patients with a lesion seen on ultrasound. An MRI-guided core biopsy can be used in situations where the lesion is occult on mammogram and ultrasound. Among women who are diagnosed with DCIS without invasive cancer on core biopsy, the estimate of upstaging to invasive cancer upon surgical excision ranges from 0% to 20% [20]. This upstaging will clearly depend on the amount of tissue provided to the pathologist and the accuracy of radiological sampling any areas of particular concern; the use of larger bore vacuum-assisted biopsy (VAB) needles accurately delineated the presence of DCIS alone in one small series [21], but in most, even with VAB, occult invasive disease will be missed on biopsy in approximately 5%–10% of women with a biopsy diagnosis of DCIS.
11.4.5 Pathology
The pathology of DCIS can be considered as a spectrum ranging between atypical ductal hyperplasia and invasive disease with features such as grade and necrosis reflecting the likely clinical behaviour as well as the presentation on mammography. There is interobserver variability when assigning a final pathologic diagnosis of ADH versus low-grade DCIS in some series (see ► Chap. 10) [22]. To avoid overtreatment, a more appropriate nomenclature may be to reclassify DCIS as ductal intraepithelial neoplasia (DIN) as was the idea of reclassifying lobular carcinoma in situ (LCIS) to lobular intraepithelial lesion (LIN) [23, 24]. However, this system has not achieved widespread acceptance, and cytonuclear grading is the system recommended in the USA and the UK, among others [25].
11.5 Differential Diagnosis
The pathological features of ADH and low-, intermediate- and high-grade DCIS are presented in ◘ Table 11.1. The relationship of DCIS to atypical ductal hyperplasia (ADH), lobular neoplasia and invasive disease deserves attention. Usually, diffuse-positive nuclear ER expression with contiguous reactivity throughout the entire population of atypical cells is seen in both ADH and low-grade DCIS (◘ Fig. 11.3) both of which are almost always ER positive. In addition, homogeneous absence of staining for basal cytokeratin markers such as cytokeratins 5 and 14 is also a common finding for ADH and low-grade DCIS. Thus, the distinguishing feature discriminating ADH from DCIS is that the cellular and architectural changes of low-grade DCIS occupy two or more complete membrane-bound spaces, those of ADH only one. The low-grade, solid variant of DCIS may be misinterpreted as lobular neoplasia but can almost always be distinguished definitely with E-cadherin (staining indicates ductal pathology).
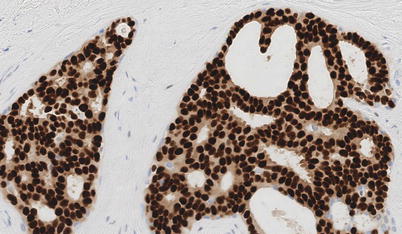
Table 11.1
Features of DCIS by grade and in comparison to atypical ductal hyperplasia
Feature | Atypical ductal hyperplasia | Low grade | Intermediate grade | High grade |
|---|---|---|---|---|
Pleomorphism | Monotonous | Monotonous | Intermediate | Markedly pleomorphic |
Cell size | 1.5 × to 2 × RBCs or normal duct epithelial nucleus | 1.5 × to 2 × RBCs or normal duct epithelial nucleus | Intermediate | >2.5 RBCs or normal epithelial nucleus |
Chromatin | Usually diffuse, finely dispersed | Usually diffuse, finely dispersed | Intermediate | Usually vesicular, regular chromatin distribution |
Nucleoli | Only occasional | Only occasional | Intermediate | Prominent, often multiple |
Mitoses | Only occasional | Only occasional | Intermediate | May be frequent |
Orientation | Polarised | Polarised | Intermediate | Usually not polarised |
Extent of lesion | Less than two complete membrane-bound spaces (or less than 2 mm in size) | Two complete membrane-bound spaces involved |

Fig. 11.3
Estrogen receptor (ER) staining of nuclei on a histological section of DCIS
Low-grade (◘ Fig. 11.2) and intermediate-grade (◘ Fig. 11.4) («low risk») DCIS may have a more indolent course than high-grade DCIS (◘ Fig. 11.5) with regard to its potential to develop invasive disease [23]. The cytomorphological appearances, the grade of the DCIS and the presence or absence of comedo necrosis (necrosis in the centre of the ducts) (◘ Fig. 11.5) are reflected in both the mammographic appearances and the subsequent disease behaviour. In addition, the presence of ER, PR and HER2 expression on immunohistological staining of the DCIS may have implications for the future likelihood of recurrence; however, the evidence is far from clear. While the microscopic appearances may vary, ultimately the behaviour of DCIS that is high grade (◘ Fig. 11.6) and ER negative and/or HER2 positive may be more aggressive when compared with that of low-risk DCIS. This is considered further below in the section on recurrence.
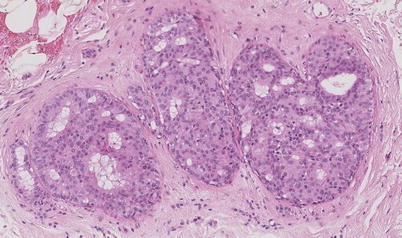
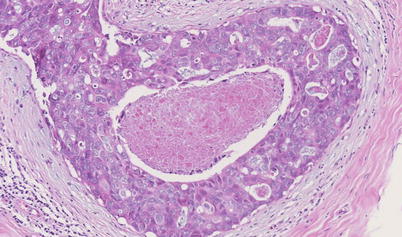
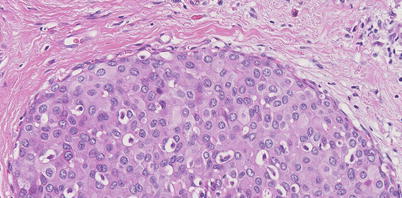

Fig. 11.4
Intermediate grade DCIS

Fig. 11.5
High-grade DCIS with central necrosis

Fig. 11.6
High-grade DCIS
11.5.1 Receptor Status
The oestrogen receptor (ER), progesterone receptor (PR) and HER2 protein status of DCIS is not routinely performed for DCIS in some European countries such as the UK where it is not included in national minimum datasets, although ER is considered a routine part of assessment in the US NCCN guidelines. Increasing DCIS grade correlates with a decrease in hormone receptor positivity; comedo necrosis is also more frequently seen in ER-negative tumours. The availability of ER, in particular, may determine the use of adjuvant endocrine therapy following a diagnosis of DCIS (see below), although the influence of this treatment is largely on the contralateral breast, and the relevance of the ER status of the index disease is not clear.
11.6 Treatment
Current treatment options routinely offered for DCIS include surgery (lumpectomy/wide excision/«segmental mastectomy» or mastectomy), radiation (radiation or none) and endocrine therapy. These options constitute guideline concordant care (GCC) according to the NCCN treatment recommendations [15]. Between 1991 and 2010, 23.8% of women diagnosed with DCIS in the USA underwent mastectomy, 43% lumpectomy with radiation and 26.5% lumpectomy without radiation, based on data from the Surveillance, Epidemiology, and End Points Registry [26]. Among the 97% of women with DCIS treated with guideline concordant care, neither randomised trials nor retrospective studies to date have shown a survival advantage of any treatment option over another [26].
11.6.1 Overdiagnosis and Overtreatment
Overdiagnosis (detection of a condition not causing symptoms or death if left undetected) and overtreatment (treatment without benefit) resulting from mammographic screening have been estimated to be as high as one in four patients diagnosed with breast cancer. [27] The absence of standard definitions for assessing overdiagnosis has led to uncertainty and controversy around this estimate. The national health-care expenditure resulting from false-positive mammograms as well as breast cancer overdiagnosis has been estimated in the USA to approach $4 billion annually [28], and there is general consensus that much of this burden derives from the treatment of DCIS. However, many fail to recognise that DCIS is not a single disease process (clinically, radiologically, biologically, histopathologically or genetically) and that low-grade DCIS and high-grade DCIS cannot be considered equivalent in terms of likelihood of progression to invasive disease or subsequent behaviour. Nevertheless, for those women whose DCIS may never progress even without treatment, medical intervention can only do harm. In addition, the potential for overtreatment and overdiagnosis must be balanced against the consequences of missed diagnosis and undertreatment [7]. Thus, at present, most women who receive a diagnosis of DCIS undergo surgical resection with consideration of adjuvant radiotherapy and/or endocrine therapy.
11.6.2 Surgery
With regard to surgical treatment for DCIS, the options comprise breast conserving surgery (BCS), with or without radiation, versus mastectomy (with or without breast reconstruction). There is no single approach that is best for every patient given the heterogeneity of disease, variation in extent, age, genetic carrier status, breast size (compared with the size of the DCIS) and the patient’s desire for breast conservation, mastectomy and/or reconstruction. Breast conservation surgery with adjuvant radiotherapy has been accepted as a treatment option for invasive disease with equivalent survival to mastectomy; this principle in surgical management has been extended to include DCIS. Since most DCIS is identified as a non-palpable lesion, BCS usually requires the radiographic abnormality to be localised by, for example, wire localisation or iodine-125-labelled seed, for resection (◘ Fig. 11.7). Determining the extent of disease is important, as large lesions may need to be bracketed with wires or seeds to ensure removal of the involved area. Assessment of the specimen radiograph helps confirm that the entire targeted lesion was removed.
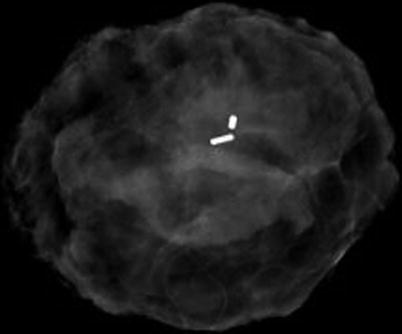

Fig. 11.7
Specimen radiography of DCIS demonstrating clip (smaller opaque marker) and I125-labelled marker (larger opaque marker); specimen orientated using sutures not visible to radiography
DCIS is most commonly unifocal. Segmental, multifocal or multicentric disease is the exception; only one of 119 mastectomies was multicentric in the series examined by Holland and Hendriks [29]. However, while multifocal or multicentric lesions are traditionally considered to be a contraindication to BCS, many groups have reported success incorporating oncoplastic reconstruction, as for invasive disease [30]. Oncoplasty has gained momentum in recent years as it may allow for wider, negative margin resection while achieving a good cosmetic result for the patient by reshaping the breast. For larger resections, this procedure can be done with a contralateral mastopexy for symmetry. Mastectomy options include a total mastectomy, skin sparing mastectomy (SSM) or nipple sparing mastectomy (NSM). Mastectomy may be the only surgical option for a woman with a small breast and large extent of DCIS. For SSM, a circumareolar incision can be modified if the patient has a scar from a previous biopsy that needs to be incorporated with the mastectomy. For NSM, an inframammary incision provides good access, although patients with documented retroareolar DCIS are not suitable for this approach. Coring out the duct tissue behind the nipple, submitted separately for pathology, can occasionally demonstrate DCIS and necessitate a return to the operating room for excision of the nipple. For DCIS, prophylactic contralateral mastectomy does not hold a survival benefit in women that do not have a defined genetic predisposition for breast cancer [31].
In those women who undergo surgical management of DCIS, there is a risk of developing persistent pain at the surgical sites [32]. Importantly, persistent pain after lumpectomy may be as prevalent as pain after total mastectomy, leading to disability and psychological distress, which is often resistant to management. Prospective population-based data have demonstrated significant patient and surgical influences on pain, with remarkably high levels of chronic pain 4 and 9 months after breast surgery [33]. Many of these data have been collected in women with invasive cancer, not purely DCIS, and so the incidence of post-operative symptoms after resection of DCIS alone is uncertain but unlikely to be very different from that of other breast surgery.
11.6.3 Margins
The goal in margin-negative resection is to remove the targeted lesion with a margin of normal breast tissue. The optimal margin width remains unclear and an area of controversy. The margin of resection required has long been debated, with the UK NICE guidelines published in 2009 [16], which recommend a minimum 2 mm radial margin for patients undergoing BCS for DCIS, concordant with meta-analysis evidence [34]. Recently, US guidelines have changed from the traditional, wider, margins to a consensus that a 2 mm margin is sufficient for DCIS [35]. Re-excision should be considered if the margin is less than 2 mm after a discussion with the patient. The British Association of Surgical Oncology recommends that units develop local guidelines. Of note, not all of the extent of DCIS necessarily bears histological (and radiological) calcification, and imaging techniques tend to underestimate disease extent in at least a substantial proportion of patients [13, 14]. Nevertheless, even with multiple bracketed localisation techniques, disease may be present beyond that anticipated. For this reason, surgical re-excision is more frequently required than for invasive disease; a retrospective study of hospital statistics in the English NHS reports 29.5% of patients with DCIS had at least one reoperation [36]. Unfortunately, most of the evidence for optimum margin width comes from observational studies. In the literature, positive margins are well accepted to increase local recurrence. The importance of positive anatomically non-breast margins (anterior/skin and posterior/pectoral fascia) remains a point of debate. If a wide local excision incorporates full thickness breast parenchyma, the only tissue anterior to the excision cavity is subcutaneous tissue and skin, which, by definition, does not contain breast parenchyma. One poll showed the variability in margin widths for surgeons in the UK and explored the different techniques surgeons use for re-excision of a positive anterior margin (scar + skin, anterior margin + skin, anterior margin + skin + adjacent tissue, all margins including skin) [37].
Importantly, it appears that margins greater than 2 mm in women treated by breast conservation and external beam radiotherapy do not confer an advantage in terms of reduced risk of recurrence. Previously, a meta-analysis of trials for the effect of margin status on local recurrence after breast conservation and radiotherapy for DCIS [34] demonstrated that negative margins significantly reduced the risk of ipsilateral recurrence when compared with a close or unknown margin (OR 0.59 and 0.56 respectively). Where margins were specifically measured, a 2 mm margin was superior to a margin of less than 2 mm (OR 0.53) but not significantly different to a margin >5 mm [34].
11.6.4 Lymph Nodes
In the UK, NICE guidelines recommend that sentinel lymph node should be considered for patients undergoing mastectomy, as this procedure cannot be undertaken subsequently if unanticipated invasive disease is detected histologically [16]. Some also consider sentinel lymph node evaluation in patients undergoing oncoplastic reconstruction where extensive tissue mobilisation is planned. If an occult invasive malignancy is found, the tissue mobilisation can interfere with the lymphatic drainage of the breast and may result in inability to accurately stage the axilla.
When local excision is performed for DCIS, SLN biopsy is possible as a subsequent procedure if invasion is identified on final pathology. In a meta-analysis [38], Ansari and colleagues reported an overall 3.7% nodal positivity rate for patients with a definitive postoperative diagnosis of pure DCIS, although a large retrospective review in the UK identified node positivity in only 0.2% of cases of screen-detected DCIS [39]. While the routine use of sentinel lymph node biopsy in DCIS has been debated, in patients with pure DCIS, lymph node status has failed to predict inferior outcomes and hence should not change subsequent management.
11.6.5 Radiation
Unlike invasive breast cancer, radiation therapy is very rarely used following mastectomy for DCIS with a rate of less than 1% [40]. The indications appear to be close margins and large tumour size, in a large national UK survey of nearly 10,000 cases of DCIS, with no recurrences at 5 years follow-up.
In contrast, the majority of women with DCIS undergo breast conserving surgery, for whom radiotherapy is offered as adjuvant therapy in 1/3–2/3 of patients, although there is low consensus as to how best to select women for adjuvant radiotherapy. Whole breast radiotherapy following breast conserving surgery (◘ Table 11.2) reduces the local recurrence by more than half from 28.1% to 12.9% and reduces the incidence of invasive disease from 11.0% to 5.0% at 10 years in meta-analysis [41] based on key randomised prospective trials [2, 42–44]. More recently, other retrospective cohort studies [45] or randomised trials of radiotherapy or not after surgery [46] of low-risk DCIS have suggested lower levels of recurrence than with surgery alone, but still a marked effect of radiotherapy (◘ Table 11.2).
Table 11.2
Recurrence of breast neoplasia with or without adjuvant radiotherapy
Study | DCIS features | Locoregional recurrence no radiotherapy | Locoregional recurrence with radiotherapy | |
|---|---|---|---|---|
Wong [45] | Retrospective cohort | ≤1 cm size >1 cm margin Grade I, II | 15.6% at10 years | |
McCormick [46] | RTOG 9804 | ≤2.5 cm size ≥3 mm margin Grade I, II | 6.7% | 0.9% |
Correa [41] | Meta-analysis | 18% at 5 years 28.1% at 10 years | 8% at 5 years 12.9% at 10 years | |
Stuart [49] | Meta-analysis | 24.7% at 10 years with tamoxifen | 14.4% at10 years |
However, while radiotherapy halves breast recurrence, it does not appear to alter long-term breast cancer-specific survival [47]. The benefits of radiotherapy may be offset by the increased risks of lung, oesophageal and contralateral breast cancers, cardiovascular risks and, rarely, (0.1%) angiosarcoma based on historical studies of radiotherapy and DCIS [41]. While there may be no demonstrable survival advantage of breast radiotherapy, conversely there is no excess mortality from the use of radiotherapy in the setting of DCIS [41].
The survival advantages and cosmetic benefits seen for hypofractionation of radiotherapy for invasive breast cancer in large Canadian, UK and US trials may be expected to pertain to adjuvant radiotherapy for DCIS. The potential for partial breast radiotherapy (whether external beam, brachytherapy or intraoperative radiotherapy) for DCIS has not been fully explored.
11.6.6 Endocrine Therapy
There is evidence from one placebo-controlled trial, NSABP B-24, in the USA, that in pre- and postmenopausal patients treated for DCIS with lumpectomy and adjuvant radiotherapy, the addition of tamoxifen reduces the risk of ipsilateral local recurrence by 30% and of contralateral breast cancer by 50% [48]. The absolute risk at 5 years of any (invasive or non-invasive) breast cancer event was small (tamoxifen arm 8% and placebo arm 13%). Survival was not influenced by treatment. Another complex trial design examined the use of tamoxifen versus no adjuvant therapy following complete local excision of DCIS in the absence or presence of radiotherapy [42]. In the absence of radiotherapy, tamoxifen was, again, associated with a 30% overall reduction in breast events through reduction in DCIS recurrence as well as contralateral DCIS and invasive disease events. Tamoxifen was, however, ineffective in preventing ipsilateral invasive recurrence, and in the presence of radiotherapy, tamoxifen also appeared ineffective. Survival was not improved by the addition of radiotherapy or tamoxifen on top of surgery alone in this trial, with breast cancer accounting for only 20% of all deaths (2% breast deaths and 11% overall deaths) [42]. Overall, meta-analysis including these trials suggests a modest additional benefit of tamoxifen over a combination of breast conservation and breast radiotherapy for local recurrence with a reduction from 14.1% to 9.7% [49].
Recently, anastrozole, an aromatase inhibitor (AI), has been compared to tamoxifen in postmenopausal women with DCIS. In NSABP B-35 which enrolled 3104 postmenopausal women who had undergone lumpectomy with confirmed clear margins and subsequent adjuvant radiation for DCIS, anastrozole treatment was associated with a small but statistically significant improvement in breast cancer-free interval compared to tamoxifen (HR 0.73 [95% CI 0.56–0.96], p = 0.023), although disease-free survival was the same at 120 months (HR 0.89 [95% CI 0.75–1.07], p = 0.21) [50]. Among women <60 in this study (n = 1447), anastrozole was associated with significant improvements in breast cancer-free interval and disease-free survival compared to tamoxifen, with hazard ratios (HR) of 0.53 (95%CI 0.35–0.80) and 0.69 (95% CI 0.51–0.93), respectively. However, the International Breast Intervention Study (IBIS) II trial, which enrolled 2980 postmenopausal women with DCIS who had undergone lumpectomy to achieve clear margins +/− radiation, failed to demonstrate an improvement with the AI compared with tamoxifen/placebo (HR 0.89 [95% CI 0.64–1.23], p = 0.49) [51].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







