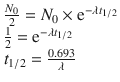(1)
Institute of Oncology “Prof. Ion Chiricuță”, Nuclear Medicine & Endocrine Tumors, Cluj-Napoca, Romania
“Multum in parvo”
– Much in little –
Latin proverb
According to our knowledge so far, the dosimetric approach in nuclear medicine is less extensive than in external beam radiotherapy. The strategy of fixed doses of radiopharmaceuticals is almost a routine in diagnosis and also in many nuclear therapeutic procedures.
During the last decade, many studies were performed on this topic, especially concerning the treatments of neuroendocrine tumors, limiting the side effects and toxicity of radiopharmaceuticals, and, in thyroid carcinoma, trying to improve the management of some radioiodine resistant forms of the disease. The most relevant issues regarding the strict calculation of doses refer to:
Decay of radionuclide and dose activity calculation for the administration
Dose rates for radiation protection
Dose rates to the target organ (the tested or treated organ) and critical organ (the most susceptible to irradiation during the diagnosis test or treatment)
Most up-to-date publications on radiation dosage of radiopharmaceuticals used to patients, during the nuclear medicine procedures, appear in the International Commission on Radiological Protection (ICRP) Publication 106, addendum 3 to ICRP Publication 53, and ICRP Publication 133. The most important dosage rates of labeled radionuclides used in human studies are listed in these publications. It is important for the clinical practice to underline that all radiopharmaceuticals used in nuclear medicine suffer decays, with halftimes varying from seconds to years. The clinician should organize the activity and the testing or treating procedures, taking into account the following special feature: the estimated dosage to be administrated may decrease until its total disappearance.
The decays of the most important radiopharmaceuticals used in nuclear endocrinology will be presented in this section of the book; these data are very important in daily practice, in order to calculate the activities and to decide which radiopharmaceutical will be used for each patient and the type of diagnostic test or therapy to be used.
Key Point
In nuclear medicine, it is mandatory to respect the physic characteristics of the radiopharmaceuticals used, and further, the time schedules of any nuclear medicine procedure became essential.
3.1 Decays of Radionuclides and Doses Calculation
The doses that need to be administered are calculated according to the purpose of the diagnosis or the therapeutic test, to the decay of the radionuclide, and to the patient’s body weight and age.
The pediatric doses are adjusted using two different methods, presented in Eqs. 3.1 and 3.2:
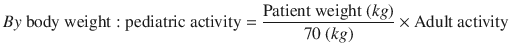
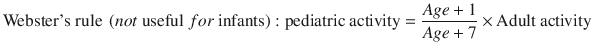
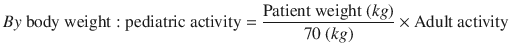
(3.1)
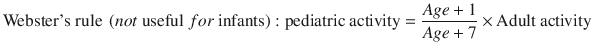
(3.2)
Some of the radioisotopes are nuclear reactor products, and others are cyclotron radioisotopes. Carbon C-11, nitrogen N-13, oxygen O-15, and fluorine F-18, these are cyclotron products and are positron emitters used in positron emission tomography (PET); they are used for studying brain physiology and pathology, in particular in dementia, psychiatry, and neuropharmacology studies. They also have a significant role in cardiology. F-18 in FDG has become essential in detection of cancers and the monitoring of progress in their treatment, using PET. In the following pages, the characteristics of the most frequently used radioisotopes in endocrinology, in practical clinic, are presented.
3.1.1 Fluorine F-18
Radiation:
Gamma: 511 keV (194% abundance), positron annihilation radiation
Beta: 249 keV (97% abundance)
Half-life (T 1/2):
Physical T 1/2: 109.8 min
Biological T 1/2: 6 h
Effective T 1/2: 1.4 h
Critical organ: lung, stomach
Exposure routes: ingestion, inhalation, puncture, skin contamination absorption
Radiological hazard: external and internal exposure, contamination
3.1.2 Gallium Ga-68 and Gallium Ga-67
Ga-68:
Radiation:
Gamma and X-rays: 511 keV (178% abundance), 1077 keV (3%)
Beta: 1899 keV (88% abundance), 822 keV (1%)
Half-life (T 1/2):
Physical T 1/2: 78 h
Biological T 1/2: 12 years (gallium citrate)
Effective T 1/2: 3.3 days
Critical organ: lung, gastrointestinal tract
Exposure routes: ingestion, inhalation, puncture, skin contamination (absorption)
Radiological hazard: external and internal exposure, contamination
Ga-67:
Radiation:
There are in principal three gamma emissions at 93, 185, and 300 keV.
Half-life (T 1/2):
Physical T 1/2: 1.13 h
Biological T 1/2: 12 years (gallium citrate)
Effective T 1/2: 1 h
Critical organ: bone marrow, gastrointestinal tract (the lower bowel)
Exposure routes: ingestion, inhalation, puncture, wound, skin contamination (absorption)
3.1.3 Radioiodine I-123
Radiation:
Gamma and X-rays: 159 keV (83% abundance), others
Electrons: 3 keV (94% abundance), 24 keV, 127 keV (others)
Half-life (T 1/2):
Physical T 1/2: 13.22 h
Biological T 1/2: 120–138 days
Effective T 1/2: 12 h
Critical organ: thyroid gland
Exposure routes: ingestion, inhalation, puncture, skin contamination absorption
Radiological hazard: external and internal exposure, contamination
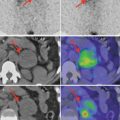
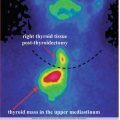
Table 3.1 presents the decays for radioiodine I-123 useful for the diagnosis in endocrinology
Table 3.1
Decays of radioiodine I-123
Hours | 0 | 1 | 2 | 3 | 4 | 5 | 13 | 15 | 18 | 20 | 24 |
|---|---|---|---|---|---|---|---|---|---|---|---|
The decay factor | 1 | 0.948 | 0.900 | 0.854 | 0.810 | 0.769 | 0.505 | 0.454 | 0.388 | 0.349 | 0.283 |
3.1.4 Radioiodine I-124
Radiation:
Gamma and X-ray: 511 keV (46%), 603 keV (61%), 1691 keV (11%), others
Beta: 1532 keV (11%), 2135 keV (11%)
Half-life (T 1/2):
Physical T 1/2: 4.18 days
Biological T 1/2: 120–138 days (unbound iodine)
Effective T 1/2: 4 days (unbound iodine)
Critical organ: thyroid gland
Exposure routes: ingestion, inhalation, puncture, skin contamination absorption
Radiological hazard: external and internal exposure, contamination
3.1.5 Radioiodine I-125
Radiation:
Gamma and X-rays: 35.5 keV (7% abundance), 27 keV (113% abundance), others
Half-life (T 1/2):
Physical T 1/2: 59.4 days
Biological T 1/2: 120–138 days
Effective T 1/2: 42 days
Critical organ: thyroid gland
Exposure routes: ingestion, inhalation, puncture, skin contamination absorption
Radiological hazard: external and internal exposure, contamination
Table 3.2 presents the decays of I-125.
Table 3.2




Decays of radioiodine I-125
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree