The diagnosis and management of severe thrombocytopenias can be difficult, but is necessary to avoid significant morbidity and mortality. The causes of severe thrombocytopenias, often with a platelet count of less than 10 × 10 9 /L, include heparin-induced thrombocytopenia, the thrombotic microangiopathies, the catastrophic antiphospholipid syndrome, preeclampsia/HELLP, and posttransfusion purpura. This review provides a brief overview of the key clinical features of each of these major clinical entities, and strategies for their diagnostic workup and therapeutic management.
The consulting hematologist is often faced with the evaluation of patients with low platelet counts, many times of less than 10 × 10 9 /L (single-digit thrombocytopenia). These consults require quick action for both diagnosis and treatment because the consequences of a severe thrombocytopenia may include life-threatening bleeding, especially intracerebral hemorrhage, or, paradoxically because of accompanying thrombosis, limb-threatening ischemia. The major causes of these severe thrombocytopenias are heparin-induced thrombocytopenia (HIT), the thrombotic microangiopathies, catastrophic antiphospholipid syndrome, and posttransfusion purpura. Immune thrombocytopenia purpura (ITP) is also a cause of severe thrombocytopenia but is described elsewhere in this issue and is not addressed here. This article covers the basic definitions, pathophysiology, and management strategies for these life-threatening and limb-threatening thrombocytopenias. More detailed discussions of each individual topic can be found in the cited primary literature and reviews.
Disease entities
Heparin-Induced Thrombocytopenia
HIT is divided into 2 major types. Type 1 HIT is benign and self-limited, and is characterized by a mild drop in the platelet count within 2 days of heparin administration that partially or completely self-corrects with continued heparin administration. This form of HIT is due to platelet clumping and is also known to occur in vitro in heparinized specimens. Type 2 HIT, however, is more serious and requires intervention on the part of the clinician to avoid serious harm to the patient. Type 2 HIT is the result of the formation of antibodies directed against epitopes on the antigen complex of heparin combined with platelet factor 4 (PF4). It is more likely to occur in women, in those receiving unfractionated heparin as opposed to low molecular weight heparin, and in surgical patients as opposed to medical patients.
The diagnosis of HIT requires clinical suspicion and is triggered by a decreasing platelet count in the setting of heparin administration. Patients with HIT classically develop thrombocytopenia 5 to 10 days after initiation of heparin therapy, and they have a drop in platelet count by greater than 50% from their baseline and a nadir between 20 and 100 × 10 9 /L. Lesser degrees of platelet drop or a nadir of less than 10 × 10 9 /L are uncommon with HIT. The major risk associated with HIT is thrombosis; in retrospective analyses, upwards of 75% of patients with HIT may develop thrombosis. Thrombosis may be the presenting sign leading to the diagnosis of HIT.
Because many of the tests used to diagnose HIT have a long turnaround time (TAT), defined as greater than 24 hours, a clinical prediction model known as the 4 Ts was created in 2006 to assist clinicians in determining the probability of HIT. This model has been prospectively validated.
- 1.
T hrombocytopenia: Two points are awarded if the platelet count decrease is greater than 50% and the nadir is 20 × 10 9 /L or more. One point is awarded for a drop in platelet count between 30% and 50% or if the nadir is between 10 and 19 × 10 9 /L. No points are included for a platelet count decrease less than 30% or nadir of less than 10 × 10 9 /L.
- 2.
T iming: Two points are awarded if there is clear onset between days 5 and 10 or by day 1 if there is previous heparin exposure in the past 30 days. One point is awarded for a decrease that appears to be between days 5 and 10 but is not entirely clear; if the onset is after day 10 or the decrease is less than 1 day after initiation of heparin administration but the prior heparin exposure was 30 to 100 days before. No points are awarded for platelet count decreases before 4 days without recent heparin exposure.
- 3.
T hrombosis: New thrombosis or skin necrosis is awarded 2 points, whereas progressive or recurrent thrombosis is awarded 1 point. No points are awarded for the absence of thrombosis.
- 4.
O T her causes for thrombocytopenia: If no other apparent cause can be found, 2 points are awarded. If there is a possible cause, 1 point is awarded and if another definite cause can be determined, no points are awarded in this category.
A composite score is calculated by awarding points in each category and then tabulating the sum. High clinical probability is defined as 6 to 8 points, intermediate clinical probability is 4 to 5 points, and low clinical probability is 3 points or less. Determination of this score allows the consulting hematologist to make recommendations for further workup and management. The calculated score has a high negative predictive value, with only 1.6% of patients with a low clinical probability having clinically significant HIT antibodies in the validation study referenced here. The positive predictive value varied in the validation study depending on the clinical context, including experience of the clinician in applying the score to an individual patient and the frequency of which unfractionated versus low molecular weight heparin is used in an institution.
For patients with a high clinical probability (score 6–8), all heparin products should be discontinued; this should include all heparin flushes and heparin-coated catheters. Thrombosis remains a risk, and patients diagnosed with HIT should be started on a direct thrombin inhibitor (DTI) such as argatroban, bivalirubin, or lepirudin. Argatroban is cleared by the liver while lepirudin and bivalirudin are cleared by the kidney, so the clinician should take liver and kidney disease into account when making treatment decisions.
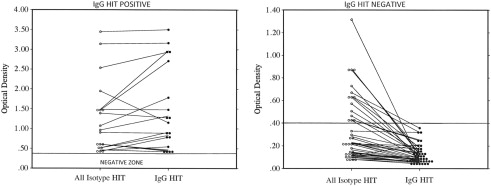
For patients with an intermediate clinical probability (score 4–5), further diagnostic testing should be conducted to either confirm or exclude the diagnosis. In the interim, all heparin products should be discontinued and a DTI initiated. Two major diagnostic tests commonly in use are a screening enzyme-linked immunosorbent assay (ELISA) to look for antibodies against the heparin-PF4 complex and a functional assay using serotonin release. If the screening ELISA is positive, a confirmatory test should be performed using neutralization with heparin or a more specific immunoglobulin (Ig)G HIT ELISA. If this confirmatory test is also positive, the patient should be considered to have HIT and treatment continued as such. A more specific serotonin release assay (SRA) can be useful, but in practice this is only performed by reference laboratories and may be limited in clinical practice because of a long TAT. A summary of the diagnostic tests for HIT are shown in Table 1 . The screening ELISA detects antibodies of all isotypes (IgM, IgG, IgA) against the heparin-PF4 complex, but only IgG antibodies are able to activate platelets by binding to the platelet FcγRIIA receptor and cause HIT. Fig. 1 shows that ELISA optical densities less than 1.0 can be shown to be negative by IgG HIT ELISA in about 50% of samples, thus avoiding the use of a parenteral DTI.
| Test | Characteristics |
|---|---|
| HIT ELISA |
|
| SRA |
|
Patients with a low clinical probability (scores ≤3) are unlikely to have HIT and, in most cases, heparin can be safely continued. Testing in this context is controversial, but a more specific test such as the IgG HIT ELISA or SRA should be used to prevent a false positive. A caveat is that there should be an ongoing assessment until a firm alternative diagnosis is made. Recalculation of the clinical probability score should be made on a daily basis with diagnostic and therapeutic interventions made as appropriate. Seemingly defying the known pathophysiology, it is possible for HIT to occur in a delayed fashion, sometimes between 9 and 40 days. Clinicians should be aware of this timing difference and consider the diagnosis.
- •
Pretest probability is determined using the 4 Ts: degree of Thrombocytopenia, Timing, Thrombosis, and oTher.
- •
Diagnostic tests include the screening (all-isotype) ELISA to detect a heparin-PF4 complex and the more specific IgG HIT ELISA or SRA.
- •
Patients considered to have HIT are at high risk for development of thrombosis if continued on heparin, so all heparin administrations should be discontinued and patients started on a DTI.
[Tags: heparin-induced thrombocytopenia, HIT, heparin, platelet-factor 4, PF4, heparin-PF4 complex, serotonin release assay, direct thrombin inhibitor].
Thrombotic Microangiopathies
Disseminated intravascular coagulation
Disseminated intravascular coagulation (DIC) is a process whereby there is an imbalance in the coagulation and fibrinolytic systems of the body, leading to inappropriate thrombin generation. It is the result of an underlying clinical condition such as sepsis, malignancy, or trauma, and determination of the underlying cause is crucial to treatment. As this process continues unregulated, there is a depletion of coagulation factors, leading to bleeding. Formation of thrombi and then uncontrolled bleeding leads to end-organ damage. Thrombocytopenia is common in acute DIC and is associated with characteristic changes in clotting factors ( Fig. 2 ). Note the extremely low fibrinogen, factor VIII:C, and factor V, which required more than 24 hours to return to normal.
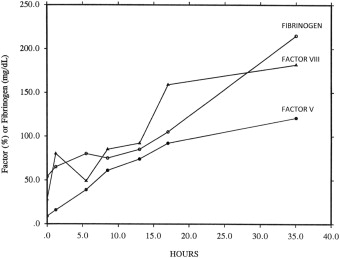
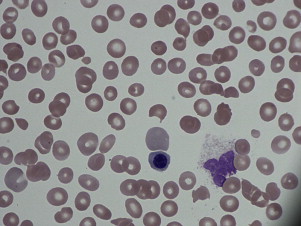
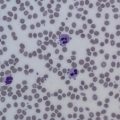
The platelet count in acute DIC is commonly less than 100 × 10 9 /L and is associated with microangiopathic changes (ie, schistocytes) seen on the peripheral blood smear. Activation of the coagulation system can be determined with widely available coagulation tests, such as decreased levels of fibrinogen, and by elevation of cross-linked fibrin degradation products, such as the D-dimer.
The most important aspect in the treatment of DIC is the identification and reversal of the underlying cause. The acute DIC process will continue until this occurs even in the setting of aggressive supportive care. Patients with a fibrinogen count of less than 150 mg/dL should have cryoprecipitate transfused because this will provide fibrinogen. There are no good data to support one serum fibrinogen target level in preference to another, and clinical scenarios should guide transfusion practice. If the prothrombin time (PT) or activated partial thromboplastin time (aPTT) is prolonged greater than 1.5 times the normal limit, fresh frozen plasma (FFP) can be transfused to replete coagulation factors. Platelets can be transfused at thresholds of 20 × 10 9 /L and red blood cells transfused to manage anemia. These criteria for blood-component transfusion are arbitrary and are intended as a guideline rather than an evidence-based approach. Clinical judgment should be used in conjunction with these laboratory tests.
Some work has been done to evaluate use of interventions such as heparin, recombinant activated protein C, thrombomodulin, tissue factor pathway inhibitor, or activated factor VII in the treatment of DIC. It should be noted, however, that there are no data to support use of these products in the pregnant patient. For women who are pregnant, standard of care remains transfusion of blood components as noted earlier.
- •
DIC is the result of an imbalance in coagulation and fibrinolysis and has several causes.
- •
Treatment involves supportive care with blood components until the underlying cause can be identified and reversed.
[Tags: disseminated intravascular coagulation, DIC, fresh frozen plasma, FFP, cryoprecipitate, schistocytes, thrombin, thrombosis, fibrin, fibrinolysis].
Thrombotic thrombocytopenic purpura
Thrombotic thrombocytopenic purpura (TTP) is the result of a deficiency or inactivation of ADAMTS13, a metalloproteinase that cleaves large multimers of Von Willebrand factor (VWF). Its deficiency leads to the presence of abnormal ultrahigh molecular weight VWF, which causes platelets to aggregate in small arterioles with resulting end-organ ischemia. A consumptive severe thrombocytopenia results, not uncommonly with a platelet count of less than 10 × 10 9 /L.
Patients often present with varying degrees of neurologic dysfunction, and on physical examination may have fever and evidence of petechiae or purpura. On laboratory evaluation there is severe thrombocytopenia, and hemolytic anemia accompanied by extreme elevations in lactate dehydrogenase (LDH). As seen in Fig. 3 , examination of the peripheral blood smear shows thrombocytopenia along with schistocytes, demonstrating a microangiopathic process. One review showed that the full pentad of microangiopathic hemolytic anemia, fever, thrombocytopenia, renal failure, and neurologic dysfunction is rare in TTP, found in only 3% of patients at presentation. Activity of ADAMTS13 can be determined and is often less than 5%, but given the TAT for this test, it is of limited clinical utility at this stage in the assessment.