Disparities in cancer care
Otis W. Brawley, MD, MACP
Overview
Differences in healthcare outcome have become increasingly apparent over the past 50 years. It is especially apparent in cancer, a discipline that has had tremendous advancement. Ironically, the more progress that is made, the greater the disparities become. There are populations that do not enjoy the progress usually because of socioeconomic differences. The field of health disparities has grown into its own discipline within epidemiology. It involves population categorization by race, area of geographic origin, socioecomic status, and so on. The discipline looks at differences in risk of cancer, risk of death, survival, and the survival experience.
A number of well-designed studies show that equal treatment yields equal outcome among equal patients whether one categorizes population by race, socioeconomics, or other major factors. If people with the same genetic markers are compared, race for example is not a factor in outcome unless it is allowed to be. Numerous patterns of care studies demonstrate that there is not equal treatment by race in the United States. The discipline of health disparities has progressed to include assessment of interventions to reduce disparities.
The advent of precision medicine and tailored therapy will make categorization using genes and polymorphisms more important. The crude categories of race and ethnicity and even socioeconomic status will only be important in terms of social issues such as access to care and equality of treatment.
Social interventions to overcome disparities and bring about equity include efforts to (1) increase cultural competence and understanding of the patient among healthcare providers; (2) increase access to care; and (3) improve communications and educate those needing service.
Introduction
Over the past 50 years, there has been tremendous progress in healthcare and especially in the prevention and treatment of cancer. This progress has led to an increasing appreciation of the differences in outcomes among populations. The first studies to discuss the fact that some populations had higher rates of death from certain cancers were conducted in the late 1960s and published in the early 1970s.1, 2 These studies noted that Black-Americans have higher death rates than Whites. The area has grown to encompass other racial groups and the poor. As the issue became more broadly understood and the discipline matured, health disparities and health equity became a political issue. Indeed, the discipline was an influence on healthcare reform legislation, as well as legislation on the design and recruitment to US government funded clinical trials.
Reporting and understanding population differences in cancer incidence and outcome is important. The causes of cancer are both genetic and environmental. When the populations and the differences between them are truly understood, reasonable hypothesis can be generated about the factors that cause or prevent cancer, as well as the factors that make cancers more or less aggressive. With rigorous careful study of the well-defined populations, one can also increase knowledge about the efficacy and effectiveness of preventive and treatment interventions.
The birth of a discipline
The US National Cancer Institute established the Surveillance, Epidemiology, and End Results (SEER) program in the early 1970s as part of the implementation of the National Cancer Act. This program collects cancer incidence and mortality data from a number of population-based registries around the United States. Before SEER, there was extremely limited cancer incidence data. Even today SEER does not provide a fully representative sample of the country for calculation of incidence rates. SEER publishes cancer incidence and mortality data as well as 5-year survival rates by race and gender annually. This data is publically available at www.cancer.gov/statistics.
Through its “Black–White Studies” in the 1980s and 1990s, SEER clearly documented Black–White racial disparities in cancer incidence, mortality, and survival. The SEER studies also demonstrated differences in treatment patterns with a higher proportion of Blacks getting inappropriate cancer care.
Interest in other diseases also contributed to the birth of the academic discipline. As cancer disparities were studied, disparities in cardiovascular disease, especially hypertension was also being defined. Interest in the genetic blood disorder known as sickle cell anemia was also increasing.
The discipline was first called “minority health research” and later “special populations’ research.” It has now evolved into the field of “health disparities” and some are now even referring to it as “health equity.” As the field has matured, the questions have become better defined and some of the solutions better elucidated. It has remained the study of the underserved, those who do not receive adequate preventive and treatment services. Today, the field of health disparities is far more than cultural competence among healthcare providers and developing specific interventions to overcome health disparities. It is transdisciplinary integrating basic science, clinical science, epidemiology, and social science.
Defining health disparities
The National Cancer Institute (NCI) defines “cancer health disparities” as adverse differences in cancer incidence, cancer prevalence, cancer mortality, cancer survivorship, and burden of cancer or related health conditions that exist among specific population groups in the United States. Translated, disparities in health are the concept that some populations do worse than others.
Factors measured
Health disparities are differences between populations. A number of outcomes can be measured and compared. Most common are as follows:
Incidence—usually expressed as the number diagnosed with the disease in a given year per 100,000 in the population. Incidence rates are the equivalent of the population’s risk of developing a disease. Individuals from groups with a higher incidence of a disease have a higher risk of getting that disease.
Mortality—usually expressed as the number dying due to the disease in a given year per 100,000 in the population. Mortality rates are the equivalent of the population’s risk of death from the disease. Individuals from groups with a higher mortality from a disease have a higher risk of dying from that disease.
Incidence and mortality are often “age adjusted” to a standard population to remove the effects of two populations having different age distributions. Age adjustment is also used to remove the effect of a population aging over time. Age is a risk factor for many cancers. A population having a higher age-adjusted incidence in 1990 compared to 1960 is not because there are more older people alive in that population in 1990 compared to 1960.
Survival rates—the median time from diagnosis to death for a cohort or the percent alive 5 years after diagnosis.
Prevalence—the proportion of a population that has a disease or risk factor for a disease.
Differences in patterns of care (screening and treatment) are also measured. This refers to the proportion of two or more groups getting a treatment standard to the condition. In more sophisticated analyses, it can be the proportion of a group getting high-quality treatment compared to another group.
Less commonly, and not in the NCI definition, disparities in morbidity, comorbid disease, and quality of life are measured outcomes.
Population categorization
While the field started by looking at Black and White race, it has evolved to the point that “populations” can be defined by race, ethnicity and culture, area of geographic origin, socioeconomic status (SES), and other factors. Clearly defining populations is extremely important in doing good health disparities research.
Race is a concept first put forth about 350 years ago. The initial categories were Caucasian, Negroid or African, and Mongoloid or Asian.3 These categories had to do with skin color, facial traits, and presumed geographic area of origin. Distinct racial groups do not exist. Based purely on visible traits, racial groups are overlapping populations. The anthropology community has never accepted race as a biologic categorization.
The US Office of Management and Budget (OMB) defines race for use in government collection of data. The OMB definition is used most importantly in the decennial census, but, by legislation, is also used to describe the populations enrolled in federally funded clinical trials.
The OMB directive specifically states that its racial categories reflect a social definition of race as recognized in the United States and that the definition is not an attempt to define race biologically, anthropologically, or genetically. The OMB racial definition has changed over time. A person from the Indian subcontinent of Asia who migrated to the United States before the 1950 census has been considered three different races over the past 65 years. The current definition was published in 1997 (Table 1).
Table 1 US Government definitions of race and ethnicity year 2000 census
| White | A person having origins in any of the original peoples of Europe, the Middle East, or North Africa. It includes people who indicate their race as “White” or report entries such as Irish, German, Italian, Lebanese, Arab, Moroccan, or Caucasian |
| Black or African American | A person having origins in any of the Black racial groups of Africa. It includes people who indicate their race as “Black, African Am., or Negro”; or report entries such as African American, Kenyan, Nigerian, or Haitian |
| American Indian and Alaska Native | A person having origins in any of the original peoples of North and South America (including Central America) and who maintains tribal affiliation or community attachment. This category includes people who indicate their race as “American Indian or Alaska Native” or report entries such as Navajo, Blackfeet, Inupiat, Yup’ik, or Central American Indian groups or South American Indian groups |
| Asian | A person having origins in any of the original peoples of the Far East, Southeast Asia, or the Indian subcontinent including, for example, Cambodia, China, India, Japan, Korea, Malaysia, Pakistan, the Philippine Islands, Thailand, and Vietnam. It includes people who indicate their race as “Asian Indian,” “Chinese,” “Filipino,” “Korean,” “Japanese,” “Vietnamese,” and “Other Asian” or provide other detailed Asian responses |
| Native Hawaiian and Other Pacific Islander | A person having origins in any of the original peoples of Hawaii, Guam, Samoa, or other Pacific Islands. It includes people who indicate their race as “Native Hawaiian,” “Guamanian or Chamorro,” “Samoan,” and “Other Pacific Islander” or provide other detailed Pacific Islander responses |
In collecting race data, the OMB has one “ethnicity” question. Ethnicity is defined by the OMB for government data as simply “Hispanic” or “non-Hispanic.”
The National Cancer Institute SEER Program and the National Center for Health Statistics of the Centers for Disease Control and Prevention publish health data using OMB definitions. They are dependent on US Census data for the denominators to calculate incidence and mortality rates. Mortality trends by OMB Race/Ethnicity Criteria as published by the NCI SEER are plotted in Figure 1. This plot uses the definition used in the 1990 census.
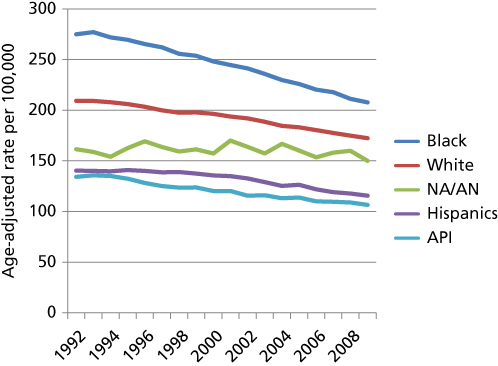
Figure 1 Age-Adjusted Cancer Mortality Rate 1990–2010 by 1990 OMB racial/ethnic category. Note: Age-adjusted mortality rates have declined significantly over the past 20 years for all but Native American/Alaskan Natives. The Native American/Alaskan Native (NA/AN), Hispanic and Asian-Islander (API) death rates are lower rates compared to Whites who have lower rates than Blacks. This graph uses the category Asian-Pacific Islander, which was the category used until 2000. Native Hawaiians are included in the Native American/Alaskan Native Category.
Race is a useful category to collect data if viewed as a social definition. Some populations do bear an unequal burden. There are civil rights issues such as discrimination and differences in access to care that determine outcomes.
Ethnicity and culture as used in academic study is a very broad term that encompasses human identity. It is not static or mutually exclusive. It is fluid and without definite boundaries. In social research, ethnic groups are distinguished by specifying the nature and source of human variation (e.g., behavior, lifestyle habits, and other environmental influences) and their relationship to health. When used appropriately, populations of one nationality can have a number of ethnicities.
This is a more scientific categorization than race as it relates to environmental influences such as diet and other extrinsic influences that may increase or decrease the likelihood of an illness. Even such habits as how one smokes cigarettes or engages in sexual activity are influenced by ethnicity and culture.
While ethnicity and culture is related to factors that cause cancer, there are also ethnic and cultural influences on ones acceptance of a disease and in how one seeks and accepts therapy.
Area of geographic origin is more scientific than race. Many confuse race and area of geographic origin and there is some overlap. Many people have several areas of geographic origin because of the commingling of populations over the centuries.
A number of genetic traits correlate with area of geographic origin. For example, sickle cell disease is a genetic disease of people from the Mediterranean basin and sub-Saharan Africa. There are people from Spain, Italy, Greece, Syria, Turkey, and Lebanon with Sickle cell disease. Natives of southern Africa do not have sickle cell disease. There are people who we consider White who get sickle cell disease and people who we consider Black who do not.
While a genetic trait can have a higher prevalence among people from a specific geographic area, its important to realize while the prevalence can be higher, the people of that area likely do not monopolize that trait. For example, cystic fibrosis is more common among, but not exclusive to those originating in northern Europe and alcohol dehydrogenase deficiency is common, but not exclusive to those from Japan.
Ancestry is yet another way of categorizing populations. Ancestry is of course linked to race and area of geographic origin. Use of race can be especially problematic as people transcend racial boundaries. When one considers ancestry, it may be a bit easier to appreciate the effect of family and admixture. Genetic influences often parallel ancestry.
Some of the Black–White differences in breast and prostate cancer might be due to differences in genetics that better parallels ancestry or area of geographic origin more so than race.
SES is defined by education, income, and occupation. While the social determinants of health are extremely important, there is controversy over how well SES can be used to define the human condition. The European literature uses a concept called “deprivation.” The “deprivation index,” calculated during the decennial census in Scotland, takes a number of markers of wealth, education, and social status into account.4
SES or social deprivation determines where we live, birthing habits, diet, and even how or if we consume healthcare. Education is particularly important, Americans with the least education are more than twice as likely to die of cancer as compared to the most educated.5–7
There can also be population differences in stage at diagnosis and differences in the distribution of pathologies by SES. Differences in the uptake of screening by SES can lead to a higher proportion of the wealthy being diagnosed with early-stage disease when compared to lower SES cohorts. A group of people who participate in screening and indeed are more likely to get high quality screening are more likely to do well. In a disease with substantial overdiagnosis, the differences can be even more dramatic.
Relationships among population categories
Race is perhaps better seen as a characteristic and not a category or subtype. Much emphasis is placed on racial groups and differences in outcome among racial groups. The emphasis is partly due to the American obsession with race and the fact that most American data on population differences is collected based on the US government definition of race. Outcomes data based on ethnicity, SES, or ancestry is not easily obtainable. While the racial categories are not based in biology, they do have some relevance for what they are, a sociopolitical construct that can be related to ethnicity, area of geographic origin and SES.
Unfortunately, the medical literature is filled with the “medicalization of race.” There are a number papers to suggest that genetics defines differences among the races and these differences are the cause of a number of disparities.8 Some of these papers should be discussing ancestry, which is different from race, and others often ignore the fact that there are clear correlations between race and SES. Low SES can be a cause of disparity. Race can also correlate with ethnicity and culture, which leads to behavior. Some behaviors increase risk of disease.
While there can be a correlation between race and cancer outcomes, it is important to remember that correlation does not necessarily mean causation. Racial differences are often due to social issues such as discrimination in education and in the provision of healthcare. Many in medicine falsely consider race a biologic categorization, thinking of race to be defined by genes. Sickle cell disease is a genetic, inherited condition. In the United States, it was and is still wrongly viewed as a “Black disease.” This helped reinforce many of the views regarding race, genetics, and risk of disease. We often hear about race as a biologic category influencing disease in the discussion of prostate cancer incidence and mortality or in the discussion of the higher prevalence of triple negative breast cancer in Black women compared to Whites.
There is a lack of well-defined universal terminology in describing the above-mentioned categories making it difficult to assemble information on disparate/underserved populations. SES often includes education and income and even measures of wealth, but neglects to capture status in the social hierarchy. The European social science concept, deprivation and the deprivation index, takes social hierarchy and SES into account. It is a more precise tool than SES for measuring social situation.
Even using the crude measure of SES, there are clear correlations between race and SES. As a result, the effect of SES on risk of a bad health outcome is often and can easily be misattributed to race. Indeed, researchers often toss a SES measure into a model without considering the potential cause or contributory role or conclude that a racial/ethnic difference must be genetic or cultural after one or two SES measures are controlled for.
SES is often correlated with a higher risk of certain cancers because the poor have a higher prevalence of habits associated with disease risk. These habits are often due to poverty. For example:
- High caloric intake, lack of physical activity, and obesity are habits causally associated with more than a dozen cancers.9 It is estimated that this triad is responsible for nearly a third of all cancers in the United States and it is a growing problem internationally. Those with lower SES in the United States have a higher prevalence of obesity.10 The less educated and poor do tend to consume more calories per day. Processed calorie dense high-carbohydrate foods are less expensive than low calorie protein-rich foods.11
- Lung cancer and a number of smoking-related cancers are more common in the poor versus the middle class. Adults living below the poverty line are more likely to smoke compared to adults in America’s middle class (30% prevalence vs 6% of Americans with a college degree).12
- The poor or those of lower SES are less likely to engage in medical preventive services. Surveys show that they are less likely to receive commonly accepted screening for breast or colon cancer.13
SES, ethnicity, and culture can come together to increase cancer risk. It can lead to a cancer causing infectious disease to be endemic within a group of people, however defined. This can lead to increased risk of a cancer in that group. This has been seen in cervical cancer owing to human papilloma virus, especially in people lacking access to preventive services. The increased risk of hepatoma among people from southeast Asia is another example. It is linked to hepatitis B that has a high prevalence in that population.14 There are also studies to show that migration of cohorts from areas of low cancer risk in Asia to and acculturation in the United States increases risk of cancer within two generations.15
Disparities in treatment patterns
Many of the early health disparities studies focused on the fact that there are significant disparities in the quality of treatment received. In the initial studies, a higher proportion of Whites receive high-quality treatment compared to Blacks. Studies suggested that a number of factors lead to differences in treatment received.16, 17 Poverty, lack of insurance, or social disenfranchisement puts a patient at higher risk of receiving poor-quality healthcare or being underserved.18
Some patients:
- Decline therapy due to their culture discouraging acceptance or cultural differences with the healthcare provider.
- Do not adhere to prescribed regimens due to illiteracy or lack of medical sophistication.
- Do not adhere to prescribed therapy due to inconvenience of getting care.
- Cannot receive preferred more aggressive therapies due to comorbid diseases more common in disparate populations.
- Are not offered needed care due to racism or discrimination based on SES.
Awareness of these issues is extremely important and the biggest hurdle to overcoming them. Healthcare providers sensitive to these issues are often able to provide better service.19 There has been a move toward training physicians to provide “culturally competent” care.20 The American Society of Clinical Oncology and others offer courses on communicating to patients from backgrounds different from the provider www.university.asco.org/cultural-competence-oncology-practice.
The fact that some groups are more likely to receive poor quality of treatment has been most rigorously studied in breast cancer. Lund et al.21 studied treatment patterns in metropolitan Atlanta over a 2-year period (2000 and 2001). Assessing women diagnosed with a localized breast cancer, they found that 7% of Black and 2% of White received no therapy within the first year after diagnosis.
Others have demonstrated that minority race, lack of education, lack of insurance, and lower SES are variables predicting for patients more likely to get inadequate care.17, 22 When these studies are assessed with logistic regression, regardless of race, less educated breast cancer patients are more likely to receive nonstandard breast cancer treatment regimens and less likely to receive adequate chemotherapy dosing.10, 23 Obese breast cancer patients are also less likely to receive adequate dosing of chemotherapy.
Disparities in quality of care lead to disparities in prognosis and treatment
While race does not appear to be the direct cause of these disparities in care given, race does translate into a higher proportion of Black or African-American Women receiving poor cancer treatment. This is because compared to Whites, a higher proportion of Black or African-American women have less than a high school education, and a higher proportion are obese. Approximately, 50% of Black women are obese compared to 34% of Whites. The disparate treatment of the less educated, the poor and the obese translates into a higher proportion of Blacks receiving less than optimal care compared to Whites. Again, the difference between correlation and causation is important.
Variable access to quality care by race can lead to differences in outcome in unsuspecting ways and lead to erroneous assumptions. For example, there is a Black–White disparity in American colorectal cancer death rates. Blacks have higher mortality rates and inferior 5-year survival rates when compared to Whites at each stage. This has led some to suggest that colorectal cancer is more aggressive in Blacks. It should be noted that Black–White colorectal mortality rates were very similar in the 1970s and the disparity in death rate has increased every year since 1981. The mortality disparity is greater in 2011 than at anytime despite the decline in mortality rates for both races.
Patterns of care studies suggest that Blacks with stage 3 disease are less likely to receive adjuvant chemotherapy and part of the disparity in stage 3 disease might be due to disparities in treatment. One might be tempted to stop here, but looking further, there are studies to show that Blacks tend to be treated in hospitals that are overcrowded and resource limited.24 They are more likely to get fewer lymph nodes resected at surgery and they are less likely to have a thorough pathologic examination of those nodes. Translated, a proportion of the Blacks diagnosed with stage 1 and 2 cancer would have been diagnosed with stage 3 colorectal if they had received appropriate surgery and pathologic evaluation. These true stage 3 patients would improve the survival rates of Black stage 3 patients by their inclusion and improve the survival of Black stage 1 and 2 patients by their exclusion.
This is a classic Will Rogers’ phenomenon.25 Blacks of each stage have inferior 5-year survival because of disparities in treatment and staging. When assessed in an equal access system, racial differences in outcome decrease dramatically.26
Genetic expression—race, ancestry, ethnicity, and culture
Still within colorectal stage with good treatment, there are disparities in outcome. Black survival is slightly inferior to White survival. EMAST, a marker of microsatellite instability, confers a poorer prognosis and is more common among Blacks or African-Americans. EMAST appears to be an acquired defect associated with inflammation.27 The role of diet, microbiome, and accumulation of EMAST is an important issue when studying Black–White colon cancer disparities. This is an example of diet, an element of ethnicity and culture, paralleling race or ancestry and affecting the genetic expression and biologic behavior of a tumor.
Some extrinsic influences associated with race, ethnicity, and culture and even socioeconomics can affect genetic expression within a malignancy. A higher proportion of American White women are middle class and college educated. Middle class, college educated women often delay childbirth to establish a career. Having a first term pregnancy after the age of thirty is a risk factor, not just for breast cancer, but estrogen receptor positive breast cancer. Indeed, this is likely the reason that White American women have a higher incidence of breast cancer compared to Black American women. This is also the reason that White women have a higher incidence of estrogen receptor positive disease.28
Studies of White breast cancer patients in the United States and Scotland suggest that middle class social status in childhood is associated with a higher risk of estrogen receptor positive breast cancer in adulthood. Poverty in childhood is associated with estrogen receptor negative disease in later life.29, 30 Differences in diet and patterns of weight gain in childhood are thought to be causal. Duration of breast feeding, number of children breast-fed, and length of breast feeding have all been correlated with a lowered risk of basal-like breast cancer, but not a reduced risk of better prognosis luminal A disease.31
In the United States, the Black and Hispanic female population has disproportionately high number who are overweight and obese. This may be due to ethnicity and culture as well as SES. Obesity, or more specifically weight gain in adulthood, is thought a risk factor for postmenopausal breast cancer.32 Differing pathologic trends seen in Black and White American women may be due to these influences.31
The understanding of the environmental influences associated with SES, ethnicity, and culture in the groups being compared can be especially useful. An understanding of the genetic polymorphisms and mutations (markers) that are associated with area of geographic origin and conserved because of ethnicity and culture is also helpful. As the field of epigenetics progresses, the affect of extrinsic influences will be even better appreciated.
Population and genetic differences
While evidence suggests that differences among broad ill-defined population categories have been overemphasized, there are intrinsic genetic differences between well-defined populations. Race is not the appropriate way to define those populations. Intrinsic genetics markers better correlate with ancestry, area of geographic origin, and sometimes with ethnicity and culture. Genetic differences correlated or associated with race should most often be considered familial or ancestral. A gene or series of genes can be conserved among families. Even then, the prevalence of a polymorphism or gene may be higher in a specific population, but that population is unlikely to monopolize that gene.33
A closed society will conserve genetic traits within that society. Segregation on the basis of race, ethnicity and culture, area of geographic origin, or other factors can lead to preservation of a specific gene or series of genes in the segregated population. This is demonstrated in several genetic diseases such as Tay Sachs disease, cystic fibrosis, and sickle cell disease.34 Each of these diseases has a higher prevalence in, but is not exclusive to, a specific group.
Perhaps the best example is that of BRCA mutations. Women with certain mutations of the BRCA-1 and BRCA-2 genes are at higher risk than average risk for the development of breast and ovarian cancer. Mutations of BRCA-1 and BRCA-2 have been found in women of all races, but three specific mutations are common, but not exclusive, to people who identify themselves as being of Ashkenazi Jewish ancestry.33, 35 Population modeling suggests that these specific mutations are linked to a small number of individuals living about 1000–1200 years ago. These mutations are considered ancestral and are common among Jewish families owing to ethnic segregation among families.
Pharmacogenomics
There is clinically relevant variability in drug response due to differences in the enzymes that metabolize drugs. These differences can vary by populations as categorized by race, ethnicity, ancestry, and area of geographic origin or even at times SES. Extrinsic factors such as diet and use of some medicines can up or down regulate hepatic enzyme expression. The same pathways through which drugs are metabolized are often involved in the detoxification of environmental toxins and carcinogens, and thus variations in detoxification enzymes may lead to variations in cancer risk.
The physician is, of course, interested in how his or her individual patient metabolizes drugs to be prescribed. One can sometimes use a form of “population profiling” to assess an individual for certain common drug treatment issues. For example, approximately 10% of persons from certain areas of Asia develop severe cutaneous adverse reactions such as Stevens–Johnson Syndrome, when administered carbamazepine.36 This might be a reasonable justification for avoiding use of carbamazepine in people whose ancestry includes an area of geographic origin in Malaysia, Singapore, Thailand, or India. If carbamazepine must be used, careful monitoring or testing for certain allele frequencies of HLA-B*1502 might be in order.
Tacrolimus has been well studied in renal transplant patients. This drug, which is structurally related to several anticancer drugs, is metabolized by CYP3A. In clinical use, some Black or African-American kidney transplant patients require higher tacrolimus doses to reach trough concentrations similar to those observed in White patients. This is due to differences in polymorphisms of CYP3A. Even within a specific group, there can be substantial variability in pharmacology. Within Blacks, there is a three to fivefold range in 12-h postdose tacrolimus concentrations.37 Special attention to pharmacokinetics is needed in all patients treated with this drug.
Among cancer drugs, population differences of polymorphisms of UGT1A1 affect the dosing and efficacy of irinotecan.38 Indeed, some suggest pharmocogenetic testing before use of this drug. Differences in ABCG2 affect the dosing and efficacy of topotecan, irinotecan, mitoxantrone, doxorubicin, and methotrexate.36
This is not a new concept, indeed in the genomic age, it is an old concept reapplied. For decades, we have appreciated that glucose-6-phosphate dehydrogenase (G6PD) deficiency is more common among people whose ancestry includes people of Mediterranean or Africa origin. Indeed, it is the most common human enzyme defect. Those with the deficiency are at risk of hemolysis when taking sulfa antibiotics, antimalarial, or certain other drugs.
US Government rules on minority inclusion in clinical trials
The US Government rules are the result of concerns that “racial minorities and women do not benefit from federally sponsored research because they do not participate in it.”
The clinical trialist doing research with funding from the US National Institute of Health must report the race and gender of patients accrued to trials on an annual basis. While the NIH is legally obligated to require this information for all phase 3 clinical trials, NIH and other government agencies often require this information for all their trials involving human subjects.
The NIH revitalization of 1993, Public Law 103-43, mandates inclusion of women and minorities in clinical research. Specifically, it states, “the Director of the NIH shall ensure that trials are designed and carried out in a manner sufficient to provide valid analysis of whether the variables being studied in the trial effect women or members of minority groups as the case may be differently than other subjects in the trial.” The stated goal of the legislation is to increase the opportunities for obtaining critically important information with which to enhance health and treat disease among all Americans and to detect and account for significant differences between genders or racial and ethnic groups where they exist and to identify more subtle differences that might warrant further exploration in targeted studies.39
The Revitalization Act is interpreted as demanding diverse representation on Federally sponsored trials, especially phase III trials. The funded researcher must make a good faith effort to accrue minorities and women proportionate to the US population.39
The legislation is controversial in that it is scientifically flawed. It calls for subset analysis to assess racial and ethnic differences. It uses race and ethnicity as if they define biological categories.39 An additional issue is the legislation calls for subset analysis. A cardinal rule of clinical trialist is subset analyses are often wrong and should only be used to establish a hypothesis to be tested in a more rigorous study. A subset analysis is post hoc, retrospective, and underpowered. Power could be increased by oversampling minorities, but this creates ethical concerns in that a disproportionately greater number of minorities would be subjected to the risk of the study compared to majority Whites.
Interestingly, the law was written after a subset analysis of a study suggested that zidovudine was less effective among the Black or African population.40, 41 The result is many Blacks with HIV stopped taking or refused to start antiviral therapies. It was not widely appreciated that later studies showed that equal treatment yielded equal outcome among equal patients. A higher proportion of Black participants in the original study started therapy with more advanced HIV disease and were less likely to adhere to the prescribed regimen for social reasons.42 Factors associated with SES again causing a difference in outcome which some interpreted as due to an inherent racial-biologic difference.
The lawmakers who wrote the NIH Revitalization assumed that disparities exist because the drugs and therapies used in the treatment of major diseases have not been tested in minorities and women. Some actually believed that disease like cancer are different in the different races. Other believed that drugs or therapies have different affects in Blacks versus Whites.43 Ignored is an important fact; many of the racial differences are because minority and poor patients do not get the treatment that is known beneficial to them and treatments not administered certainly do not work.
If health disparities are to be overcome and we are to have equity, it is best to embed good disparities related research questions into cancer treatment, prevention, screening, and control trials. This can provide more robust statistical power to address important questions.
Diverse enrollment in clinical trials is important. In the study of interventions and outcomes, there is the concept of efficacy and effectiveness. Efficacy is how well an intervention works in an ideal clinical environment. Effectiveness is how well that intervention works in a “real-world” situation. It is important that persons from all communities participate in clinical trials. An effort to do research in the community where the majority of cancer care is provided is an effort to make results more broadly pertinent. Study of accrual to NCI funded treatment studies has for some time shown relative racial/ethnic balance in clinical trial enrollment and refusal rates.44, 45 Indeed, there is interest in inclusion of the elderly, people with comorbid diseases in clinical trials to get more realistic findings.46
Summary
Health disparities or health equity research and programs are important. It is paramount that we carefully define our measures of outcome and the populations being compared. There are differences in disease incidence and outcome by population, however defined.
An important fact is a number of well-designed studies and meta-analysis of studies show that equal treatment yields equal outcome among equal patients.26 If people with the same genetic markers are compared, race is not a factor in outcome unless it is allowed to be. Rarely discussed is the fact that numerous patterns of care studies demonstrate that there is not equal treatment. Often find the discussion focuses on is a particular breast cancer drug is as effective in Blacks as it is in Whites, but it is forgotten that a substantial proportion of Blacks do not get adequate treatment to include surgery as well as chemotherapy and radiation.
With scientific progress, our understanding of cancer improves. We better appreciate its causes, biologic behaviors, and treatments. We are quickly moving into an era of personalized medicine in which genetics and genomics become very relevant. The advent of precision medicine and tailored therapy will make categorization using genes and polymorphisms more important. The crude categories of race and ethnicity and even SES will only be important in terms of social issues such as access to care and equality of treatment.
Social interventions to overcome disparities and bring about equity include efforts to:
- Increase cultural competence and understanding of the patient among healthcare providers
- Increase access to care
- Provision of insurance
- Staffing community health centers
- Attention to the patients social situation
- Provision of insurance
- Improve communications and educate those needing service
- Targeted messaging
- Patient navigation
- Targeted messaging
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree