Diarthrodial Joints
Thomas V. Smallman
Kris Shekitka
Donald Flemming
In tribute to the late Donald E. Sweet, Director of Department of Orthopaedic Pathology, Armed Forces Institute of Pathology, Washington, DC.
The objective of this chapter is to review briefly the development of joints and their normal structure and function and then to outline concisely the pathophysiology of arthritic disorders. Infectious arthritis is fully covered in Chapter 27, Infectious Disorders of Bone and Joints, and is not discussed here. The structure and function of hyaline cartilage and the cell biology of cartilage repair are covered in Chapter 20, Articular Cartilage Structure, Composition, and Repair. The reader might wish to review the key elements in this chapter prior to studying this section.
Background
The articular system consists of:
Diarthrodial joints: mobile joints consisting of hyaline cartilage covering juxtaposed bone ends surrounded by a capsule, the innermost layer of which is synovium
Factors to consider in joint motion
Joint geometry
Loading environment
Soft tissue and articular surface load sharing
Loading is accomplished through deformation of the whole joint complex.
Amphiarthroses: less mobile structure consisting of a fibrocartilaginous plate between two bone ends covered by hyaline cartilage with an intervening “disc” material surrounded by a fibrous capsule
Examples include the symphysis pubis, the posterior superior two thirds of the sacroiliac joint (anterior inferior third is synovial), and the intervertebral disc (see Chapter 23).
Nature of intervening material determines the degree of motion achieved.
Syndesmoses: nonmobile junction between bone ends, consisting of a simple fibrous connection
Examples include the distal tibiofibular joint and the skull sutures.
Disorders of joints are numerous. The essential steps of history, physical examination, problem definition, and investigation will usually lead the clinician to an ordered approach, a listing of options that leads to further inquiry, and usually an ultimate diagnosis. An understanding of normal growth and development of joints provides a basis on which to develop models for the pathophysiology of disease.
Development of Diathrodial Joints
(Limb bud development is reviewed in Chapter 14, Growth and Development of the Musculoskeletal System.)
Box 21-1 Classification of Joint Disorders
Osteoarthritis
Primary
Secondary
Inflammatory Arthritis
Rheumatoid arthritis
Juvenile rheumatoid arthritis (Still’s disease)
Spondyloarthropathies
Ankylosing spondylitis
Reactive arthritis
Psoriatic arthritis
Arthritis associated with inflammatory bowel disease
Undifferentiated
Infectious Arthritis
Bacterial
Granulomatous (tubercular, fungal, sarcoidosis)
Lyme arthritis
Metabolic
Gout
CPPD crystal deposition disease
Ochronosis
Hemophilia
Circulatory Disorders Affecting Joints
Neuropathic arthropathy
Avascular necrosis
Anomalies
Epiphysealis hemimelica
Beginning during the sixth week, condensed cells in central mesenchyme within each limb bud become chondroblasts, making very primitive cartilage, so-called cartilage models of each bone.
By end of the eighth week within each limb bud, beyond the central cartilage, mesenchymal cells continue to transform into all elements of the limb (skeletal muscle, ligaments, tendons, fat, vascular elements).
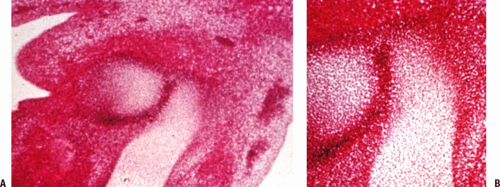
Dense interzones, darker areas between the chondrified bone models, appear at 6 weeks and will become the joints (Fig. 21-1).
Cavities within interzones appear at 10 weeks (Fig. 21-2).
Mechanism of cavity formation: genetically determined enzymatic destruction with or without simple elaboration of synovial fluid; does not require activity of muscles, often occurs before adjacent muscles have formed or attached
Persistence and maintenance of joints, once formed, require muscle action. Formation of the unique anatomy of each joint unfolds locally.
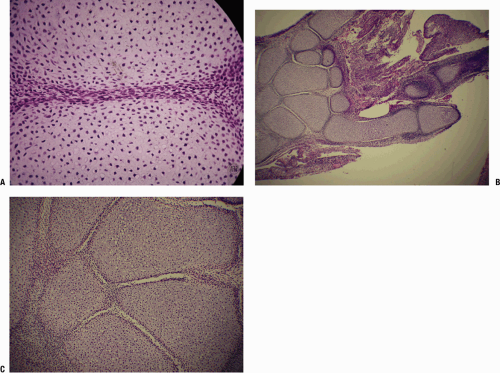
All of the knee structures are present at 14 weeks. Menisci form as fibrocartilaginous semicircles with peripheral attachment; at no time in normal development are they complete discs separating the bone ends.
All elements of the hip are formed at 4.5 months.
Normal Structure and Function of Joints
Homeostasis in a diarthrodial joint involves important relationships between the three key components of the joint: articular cartilage, synovium, and subchondral bone.
Three important vectors of circulatory, mechanical, and metabolic influences are in constant interplay at any given time, affecting each of these components.
Articular Cartilage
Articular cartilage is aneural and avascular, with diffusion from synovial fluid through the extracellular matrix being the route for its nutrition.
Chondrocyte mediates all processes for articular cartilage: synthesis, maintenance, degradation, and repair.
Repair is problematic (see Chapter 14) because the replacement material that results from the repair process lacks the normal architecture of the articular cartilage (see Fig. 20-1A in Chapter 20).
Each joint is unique, reflecting its biomechanical tasks of motion, weight transmission, and stability. Inherent in the structure of each joint is conforming bony and articular surface anatomy, the synovial lining layer, ligaments and capsule with unique local geometry determined by the motion required, allowing recesses for accumulation and disposition of debris, and specialized fluid transport channels. The joint remodels throughout life, shedding material and cells from the surface of the articular cartilage, and replacing these cells with those arising from a growth zone located adjacent to the tide-mark.
Motion and activity within physiologic limits are essential. An appropriate exercise pattern throughout life promotes through Wolff s law the development and maintenance of optimal structure of bone, articular cartilage, ligaments, muscles, and tendons.
Synovium
The role of the synovium is to maintain joint homeosta-sis and to provide the appropriate response in disease states. Synovium differs from true epithelium because it has no basement membrane, thus facilitating exchange of nutrients and other substances between the vascular system and the joint fluid.
Three histologic types are described:
Areolar synovium
Structure
Surface compact layer of collagen (types I and III) and synovial cells
Deeper layer is of fibrovascular matrix, vessels, nerves, migratory cells (mast cells and mononu-clear cells), and ground substance, including dermatan sulfate and hyaluronic acid.
Three cell types exist:
Phagocytic cells (type A synoviocytes)
Maintenance cells (type B synoviocytes)
Intermediate forms (type C synoviocytes)
Fibrous synovium
Present in transition zones adjacent to ligament surfaces
Provides a conduit for neurovascular elements of the ligament
Structure: surface compact zone denser and more flattened adjacent to the collagen lining; surface can be discontinuous
Fatty or adipose synovium
Prototypical location is the fat pad of the knee; acts as mobile packing tissue, changing shape to match joint motion
Structure: Surface layer is collagen, surface lining cells, and neurovascular elements forming a continuous array with only a few very small gaps a few microns wide.
Deeper layer is fat and fibrous septa containing occasional synovial cells, vessels, and nerves.
Function of the fat pad of the knee may be much more complex than simple padding and protection, and may include roles in:
Paracrine function through the elaboration of cytokines
Mechanical function to condition motion between the patella, femur, and tibia
Neural function in proprioception, or in reactive neural inflammation through substance P fibers
Subchondral Bone
Each joint is a unique engine of force transmission. Dr. Sweet used an analogy in which one can think of the articular cartilage as representing the tire, the subchondral bone the rim, and the cancellous array the spokes. Cortical bone then acts as a deformable unit, accepting force and transmitting it to the metaphyseal cancellous array, which distributes the force uniformly to the articular cartilage (Fig. 21-3).
Changes in the articular cartilage diminish its ability to absorb compressive loads, causing an alteration to the subchondral bone.
Changes in the structure of subchondral bone can result in loss of structural support, exposing the cartilage chondrocytes to forces exceeding their tolerance.
In avascular necrosis (AVN), death of a portion of the subchondral plate leads to a reactive zone of granulation tissue in which osteoclastic activity leads to a loss of the bony supporting arch for cartilage, often leading to collapse and progressive arthritis.
In osteoarthritis, subchondral bone stiffens; it is not known whether this is primary or secondary to cartilage change.
Summary
A diarthrodial joint can be considered a functioning unit, the integral elements of which are:
Articular cartilage
Synovium
Subchondral bone
Capsule/ligaments
Synovial fluid
Any arthropathy will inevitably affect each of these elements.
Beyond these inherent elements, to function appropriately, a joint requires motor support (e.g., skeletal muscle with intact innervation). Primary muscle (muscular dystrophy, inflammatory myopathies) and nerve disorders, both peripheral (nerve injury, peripheral neuropathies) and central (stroke), may lead to loss of motion and disordered joint function. In both instances, secondary atrophic changes occur in all of the elements of the associated joints; late degenerative disease may then follow. Disease in a joint leads to loss of function. Pain, instability, and loss of range of motion then lead to secondary changes in the supporting muscle groups, with disuse atrophy and weakness.
Osteoarthritis
Definition
Osteoarthritis includes a heterogeneous group of conditions of multifactorial etiology, characterized by joint signs and symptoms, which are associated with defective integrity of articular cartilage, in addition to related changes in the underlying bone.
Symptoms include aching, intermittent pain, stiffness, loss of motion, swelling (usually), and crepitus.
Epidemiology
Mechanisms of Joint Destruction
(Also see the introduction to this topic in Chapter 14.)
Pathologic Sequence
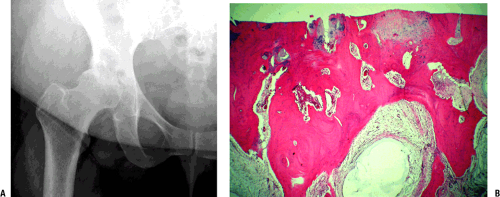
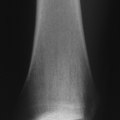
Hallmark finding (late): loss of cartilage with underlying bony changes consisting of sclerosis of subchondral bone, bone cysts, and osteophyte formation (Fig. 21-4)
The growth zone adjacent to the tidemark contributes to articular cartilage growth throughout life. Normal aging changes the contours of joints. Remodeling can add length (progressive), subtract from bone length (regressive), and add diameter (circumferential).
When the process is balanced, there is no disease.
When remodeling is unbalanced, the disease process of osteoarthritis occurs.
Early
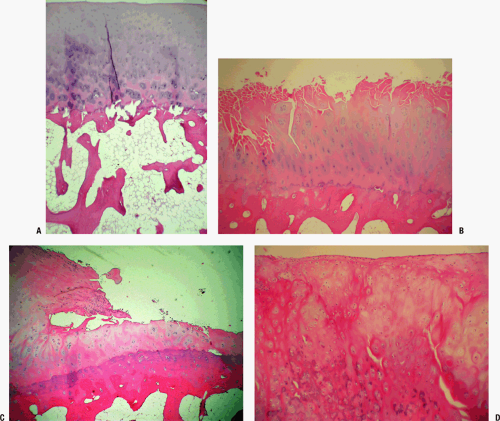
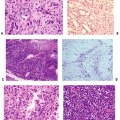
Often focal, osteoarthritis starts with superficial fibrillation and loss of small proteoglycans, decorin and biglycan (usually associated with fibrils at articular surface), and aggrecan (Fig. 21-5).
Increased type II collagen cleavage occurs by action of collagenase, aggrecan cleavage, and degradation of small proteoglycans.
Intermediate
Continued loss of proteoglycans, with increased fibrillation down to subchondral bone (see Fig. 21-5)
Increase in number of degenerating chondrocytes, with cloning and regeneration of cells
Late (see Fig. 21-4)
Mechanical abrasion of degenerative cartilage continues, with progressive loss of coverage so that the surface becomes eburnated. The loss of cartilage allows increased force to be transmitted to the subchondral plate. Increased bone formation (Wolff’s law) then occurs, and over time the subchondral bone becomes sclerotic.
Subchondral cysts develop from two potential mechanisms:
Focal mesenchymal proliferation and accumulation of “stromal mucin” (myxoid change) in marrow fat
Surface cracks allow forced entry of joint fluid into the subchondral bone.
Proliferation of tufts of metaplastic cartilage along denuded bone create a new, mechanically inferior, cartilaginous joint surface (mechanism of “healing” seen after realignment osteotomy).
Cartilage fragments shed from surface may grow as metaplastic cartilage in the joint space concentrically; this may evoke secondary metaplastic cartilaginous change and mimic primary synovial osteochon-dromatosis.
Breakdown of cartilaginous fragments may induce an inflammatory response, including chondrogranu-lomas.
Hemorrhage of friable synovium may occur, producing hemosiderin staining of synovium over time.
TABLE 21-1 Classification of Osteoarthritis
Type
Association
Presumed Mechanism
Primary
Genetic
Subtle abnormalities of articular cartilage
Secondary
Mechanical
Post-traumatic; post-slipped capital femoral epiphysis
Damage to articular cartilage; joint incongruity
Epiphyseal dysplasias
Abnormal articular cartilage; abnormal joint shape
Blount’s disease
Mechanism not known
Overuse
Damage to articular cartilage
Paget’s disease
Joint incongruity; altered subchondral bone
Joint instability (Ehlers-Danlos)
Damage to articular cartilage
Metabolic
Ochronosis; gout; CPPD crystal deposition disease
Abnormal metabolite (crystal) deposition in cartilage
Hemochromatosis
Not known
Acromegaly
Overgrowth of articular cartilage produces joint incongruity; cartilage may be abnormal.
Postinflammatory
Septic or inflammatory arthritis; hemophilia
Destruction of articular cartilage
Radiologic Findings (see Fig. 21-4)
The classic findings are of joint space narrowing (reflecting loss of cartilage), subchondral sclerosis (increased bone formation secondary to increased force across the joint surface), osteophyte formation (progressive remodeling), and subchondral cyst formation (mechanism controversial, secondary to break in subchondral bone or arising as a result of metaplasia of subchondral marrow in the environment of increased mechanical force).
Inflammatory Arthritis
Mechanism
Inflammatory arthritis is associated with specific recognition of antigenic triggers that are not fully known.
Specific immune response occurs via two cellular mechanisms and requires initiation by antigen presenting cells, which present antigens to the lymphocytes.
Humoral immunity is mediated by B lymphocytes, which produce antibodies (Ab) responsible for recognition of foreign antigens, and their elimination with the aid of complement.
Cellular immunity is mediated by T lymphocytes, which recognize and eliminate intracellular antigens.
CD4 T cells (CD4 glycoprotein surface molecule) secrete cytokines.
CD8 T cells (CD8 glycoprotein surface molecule) kill antigen-bearing target cells.
This process is governed by the MHC region of genes (HLA genes).
Cytokines
A complex network of cytokines functions in initiating and perpetuating arthritis.
These polypeptide signaling substances are produced by a host of cells, including synovial cells, chondrocytes, monocytes, macrophages, T lymphocytes, B lymphocytes, other connective tissue cells, and endothelial cells.
Rheumatoid Arthritis
Definition
Rheumatoid arthritis is an immunologically mediated inflammatory disorder of synovial joints. It can have systemic manifestations, characterized by multiple joint involvement beginning with synovitis and later arthritis that can become chronic and disabling.
Epidemiology
There is considerable variation of disease occurrence.
Highest prevalence (0.5% to 1%): Northern European, North American, some Native Americans
Intermediate prevalence (0.3% to 0.7%): Southern European
Low prevalence (0.1% to 0.5%): developing countries, rural Africa
Multifactorial disease
Genetic susceptibility
Environmental, lifestyle, and personal characteristics
Sex (female:male 2 to 3:1)
Age (onset fifth decade)
Positive factors associated with a reduced incidence
Hormonal (parity, pregnancy, oral contraceptives)
Dietary (fish, olive oil, cooked vegetables, omega-3 long chain polyunsaturated fatty acids)
Negative factors associated with an increased incidence
Smoking (increased incidence)
Infectious agents
Socioeconomic (low status worse)
Ethnic factors (higher prevalence in Northern Europe and North America)
Etiology
Susceptible individual (genetic and immunological predisposition) plus trigger:
HLA-DR4 and DW4 are most prevalent phenotypes (80%).
Trigger is unknown; possibilities are environmental antigen (infection most likely) versus nonspecific local stimuli (trauma, infection, allergic reaction, immunologic materials).
For infectious model, Epstein-Barr virus or retrovi-ruses are most likely initiators.
Pathophysiology
Regardless of trigger, IgM is produced, directed against altered autologous IgG; leukocytes phagocytose the IgM/IgG complexes along with fibrin and complement; necrosis of these cells induces release of lysosomal enzymes, which cause an acute inflammatory reaction.
Infectious trigger
Environmental antigen is attached to an antigen presenting cell macrophage and presented to T cell (CD4 phenotype is most populous in rheumatoid arthritis pannus).
CD4 + T cells produce numerous cytokines, the actions of which include:
Catabolic effects on cartilage
Activation of B cells, which differentiate into plasma cells producing IgG and IgM
Noninfectious trigger
Nonspecific stimuli induce local changes that promote the differentiation of dendritic cells in the rheumatoid synovium; these cells present an autologous Ag to the T cell, which then initiate the disease process.
Progression continues as T cells clone, resulting in recruitment of other effector cells.
Clinical Features
Diagnostic criteria are given in Box 21-2.
Typical: insidious malaise, fatigue, weakness, and vague arthralgias for weeks, followed by joint pain, swelling, morning stiffness of several joints, usually wrists, proximal joints of fingers and toes, usually symmetric distribution; any other major joint can be involved.
Pauciarticular: One third of patients have involvement of one or a few joints; then disease spreads to affect other joints, usually symmetrically.
Acute: Some patients may have an acute onset with fever and multiple swollen painful joints.
Box 21-2 Diagnostic Criteria for Rheumatoid Arthritis
|
Diagnosis
Physical Examination
Articular System
Early: warmth, tenderness, swelling with boggy feel to synovium with cutaneous ruddy cyanotic hue; muscle weakness and atrophy adjacent to involved joints
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree