Summary of Key Points
- •
In the context of disease confined to the chest, mediastinal staging is crucial for determining the best curative treatment strategy, especially for nonsmall cell lung cancer (NSCLC).
- •
Urgent referral for chest imaging is recommended for patients who present with hemoptysis or any of several symptoms or signs that are unexplained or persistent.
- •
Chest radiograph is the main radiographic investigation in the primary care setting in the diagnostic workup for suspected lung cancer.
- •
Features suggestive of intrathoracic invasion are important clues that help to distinguish between benign and malignant nodules.
- •
18 F-2-deoxy- d -glucose-positron emission tomography is an essential tool for the diagnosis and staging of disease in patients with radiographic and clinical findings consistent with lung cancer.
- •
Indeterminate nodules or negative mediastinal findings require additional procedures to obtain a tissue diagnosis.
- •
The method for definitive diagnosis and staging of lung cancer depends on the suspected cell subtype (small cell lung cancer or NSCLC), the size and location of the primary tumor, the presence or absence of radiographic findings suggestive of mediastinal involvement, and the overall clinical status of the patient.
- •
Flexible bronchoscopy should be offered to every patient with central lesions on computed tomography for whom nodal staging does not influence treatment.
- •
Transbronchial needle aspiration is increasingly being supplanted by endobronchial ultrasound fine-needle aspiration.
- •
Endoscopic ultrasound may be used to increase the number of mediastinal node stations amenable to nonsurgical sampling.
- •
Staging the mediastinum is of paramount importance in the diagnostic workup for suspected lung cancer confined to the chest, as it dictates treatment options and prognosis.
Lung cancer remains the leading cause of cancer-related death worldwide, with a 5-year survival rate of approximately 15%, as more than two-thirds of patients present with locally advanced or metastatic disease for which curative treatment is no longer feasible. Hence, the prevention and early detection of lung cancer are crucial to achieving a substantial reduction in mortality. An early diagnosis could be obtained by systematic screening of high-risk individuals or by prompt referral of symptomatic patients. Patients with lung cancer present a diagnostic challenge in that they often present with myriad symptoms and signs that are common and nonspecific (e.g., weight loss and fatigue) or that are directly related to the primary lesion, to intrathoracic spread, to paraneoplastic syndromes, or to distant metastasis. Because of this ambiguity in presentation, risk factors need to be identified in patients with a higher likelihood of having lung cancer. Smoking is the leading risk factor, but lung cancer does not develop in all long-term heavy smokers and cancer is developing in an increasing proportion of patients with no history of tobacco smoking. In fact, older age, previous diagnoses of other cancers, family history of lung cancer, and exposure to occupational carcinogens seem to increase long-term risk independent of smoking.
The initial evaluation should focus on careful physical examination and history taking to identify patients with suspected lung cancer who should have additional studies, such as a serum chemistry profile, a complete blood count, a calcium level, and testing of liver function, as well as noninvasive imaging studies such as radiographs, computed tomography (CT), and positron emission tomography (PET). According to the third edition of the American College of Chest Physicians (ACCP) evidence-based clinical practice guidelines on the diagnosis and management of lung cancer (2013), the goal of the initial evaluation of patients with suspected lung cancer is to assess key issues related to the patient’s overall health, the probability of cancer, and the probability of metastatic disease, as these factors have an impact on every other step of the diagnosis, staging, and treatment process. To optimize the management of lung cancer, the most appropriate biopsy target must be identified and any comorbidities that might limit treatment options should be addressed. The first step should be to identify whether the disease is still confined to the chest, as this factor has an impact on the need for and location of biopsies as well as on the prognosis. In this context, mediastinal staging becomes crucial for determining the best curative treatment strategy, especially for nonsmall cell lung cancer (NSCLC).
If surgery is being considered, the final component of the initial evaluation should be a physiologic assessment of pulmonary function. To stratify the patient’s operative risk, testing of lung function—specifically, measurements of forced expiratory volume in 1 second and carbon monoxide diffusion in the lung—is a helpful predictor of morbidity and mortality in those undergoing lung resection.
This chapter focuses on the diagnostic workup for suspected lung cancer that is confined to the chest and provides an extensive description of potential clinical and radiographic features as well as a practical approach to establishing the final diagnosis and staging.
Clinical Features
Symptoms related to the primary tumor include cough, breathlessness, hemoptysis, and chest discomfort. A persistent cough and dyspnea, likely due to an endobronchial mass or postobstructive pneumonia, are the most common symptoms. These symptoms occur in up to 75% and 60% of patients, respectively, and may be associated with wheezing and stridor. Intermittent, aching chest discomfort is noted by approximately 50% of patients at the time of diagnosis. Hemoptysis is rarely severe and usually only consists of blood streaking of the sputum. Forty percent of patients present with symptoms and signs related to intrathoracic spread involving the nerves, chest wall, pleura, vascular system, and/or viscera as a result of either direct extension or lymphatic spread. Recurrent laryngeal nerve palsy is more common in left-sided tumors and usually causes hoarseness. Phrenic nerve paralysis leads to an elevated hemidiaphragm and presents as breathlessness in patients who already have compromised respiratory function. A Pancoast tumor involves either the right or left apex in the superior sulcus near the brachial plexus and commonly infiltrates the eighth cervical and first and second thoracic nerve roots, causing pain, cutaneous temperature change, and muscle wasting along the relevant nerve root. It may present with Horner syndrome as a result of involvement of the sympathetic chain and stellate ganglion, causing unilateral enophthalmos, ptosis, miosis, and ipsilateral anhidrosis. Chest wall invasion can cause painful soft-tissue masses or rib destruction. Pleural effusion may be related to direct extension of the primary tumor, to implantation of tumor metastasis, or to mediastinal lymphatic obstruction and is typically heralded by dyspnea or chest pain.
Superior vena cava syndrome is the result of direct obstruction of the superior vena cava by the primary tumor or by enlarged right paratracheal metastatic lymph nodes, causing face or arm swelling; dyspnea; venous distention in the neck, upper chest, and arms; headache; upper limb edema; dizziness; drowsiness; blurring of vision; cough; and dysphagia. Lung cancer accounts for 46% to 75% of all cases of superior vena cava obstruction, and the most common histologic subtype is small cell lung cancer (SCLC).
Paraneoplastic syndromes represent a group of clinical disorders that are associated with malignant lesions but are not directly related to the physical effects of primary or metastatic tumors. Paraneoplastic syndromes occur in at least 10% of patients with lung cancer, especially SCLC, irrespective of the extension and size of the primary tumor, and may be the first manifestation of the disease. They include a myriad of endocrine, neurologic, skeletal, renal, metabolic, hematologic, cutaneous, and collagen vascular syndromes, which are likely due to the production of bioactive substances either by the tumor or in response to the tumor (e.g., polypeptide hormones, hormone-like peptides, antibodies or immune complexes, prostaglandins, or cytokines; Table 23.1 ). Hypercalcemia, the syndrome of inappropriate antidiuretic hormone secretion, Cushing syndrome, digital clubbing, hypertrophic osteoarthropathy, hematologic abnormalities, and hypercoagulable disorders are the most common syndromes observed. Nonspecific symptoms include weakness and weight loss.
| Syndrome | Type of Lung Cancer | Causative Agents |
|---|---|---|
| Acromegaly | Carcinoid tumors; small cell | Growth hormone-releasing hormone; growth hormone |
| Carcinoid syndrome | Carcinoid tumors; large cell Small cell | Serotonin |
| Ectopic adrenocorticotropic hormone (ACTH) syndrome | Small cell Carcinoid tumors | ACTH corticotropin-releasing hormone |
| Encephalomyelitis/subacute sensory neuropathy | Small cell | Anti-Hu antibody and Hu-D antigen |
| Granulocytosis | Nonsmall cell | Colony-stimulating factor (CSF); granulocyte–CSF Granulocyte-macrophage CSF Interleukin (IL)-6 |
| Hypercalcemia | Nonsmall cell (usually squamous cell) | Parathyroid hormone-related peptide; parathormone |
| Hyponatremia | Small cell Nonsmall cell | Arginine vasopressin Atrial natriuretic peptide |
| Lambert–Eaton syndrome | Small cell | Anti-P/Q channel antibody and P/Q-type calcium channel (antigen) |
| Retinopathy | Small cell | Antirecoverin antibody and specific antigen specific to photoreceptor cells (recoverin) |
| Thrombocytosis | Nonsmall cell Small cell | IL-6 |
| Thromboembolism | Nonsmall cell Small cell | Procoagulants Inflammatory cytokines Tumor interaction with host cells |
Delays in achieving a final diagnosis after the initial onset of symptoms can occur at several steps. First, the patient may notice a new symptom or a change in the usual respiratory symptoms, but some months may pass before he or she sees a physician. Additional time may then be required for the physician to obtain imaging studies of the chest, for the patient to be referred to a specialist, or for the specialist to establish a final diagnosis. Hamilton et al. evaluated the positive predictive values of symptoms and physical signs and identified so-called red flags that were independently associated with lung cancer in multivariable analyses; these red flags included hemoptysis, anorexia, weight loss, fatigue, dyspnea, persistent cough, chest pain, and digital clubbing.
According to the National Institute for Health and Clinical Excellence (NICE) clinical guideline on the diagnosis and treatment of lung cancer, an urgent referral for chest imaging is recommended for patients who present with hemoptysis or any of several symptoms or signs (including cough, chest and/or shoulder pain, dyspnea, weight loss, hoarseness, finger clubbing, features suggestive of metastasis from a lung cancer, and cervical and/or supraclavicular lymphadenopathy) that are unexplained or persistent (lasting for more than 3 weeks). Smokers and ex-smokers who are older than 40 years of age and have persistent hemoptysis, signs of superior vena cava obstruction, and stridor should be offered an urgent referral to a member of the lung cancer multidisciplinary team, usually the chest physician, while awaiting the results of chest imaging.
History
History taking should focus on major baseline risk factors, including smoking, occupational exposure (mainly to asbestos), family history of lung cancer, previous diagnosis of other malignancy, preexisting nonmalignant lung diseases (e.g., chronic obstructive pulmonary disease, idiopathic pulmonary fibrosis, tuberculosis, and previous pneumonia), and socioeconomic deprivation. Finally, residence in or travel to an area with endemic fungal pathogens could suggest a benign infectious disease in the correct clinical context.
Imaging Features and Diagnostic Accuracy
Radiography plays a crucial role in the diagnostic workup for suspected lung cancer. The main investigation in the primary care setting is the chest radiograph. The radiographic appearance of lung cancer at the time of the initial presentation may vary. Lung cancer occurs more often on the right side rather than on the left side and in the upper lobes rather than in the lower lobes, with a predominance in central locations. Up to 40% of the radiographic findings associated with lung cancer are related to central tumors causing airway obstruction with secondary atelectasis and lung parenchyma consolidation. However, although a radiograph of the chest may lead to the identification of a suspected lung mass, it lacks sufficient resolution to differentiate benign from malignant disease, and, if a previous radiograph is not available to demonstrate stability over 2 years, the patient will need additional evaluation. A negative result does not exclude lung cancer, especially if there is a high pretest probability. Stapley et al. retrospectively reviewed the medical records of 247 patients with lung cancer to assess radiographic findings in the primary care setting and reported that more than 10% of patients had had negative radiographic findings in the 3 months before diagnosis. Moreover, negative findings on chest radiographs may occur with any cancer symptom other than hoarseness. Therefore the standard imaging study for patients with suspected lung cancer is conventional contrast-enhanced CT of the chest, as it provides anatomic details such as the location, shape, margins, and attenuation characteristics of the primary lesion; the proximity of the lesion to surrounding structures; the extent of invasion of the chest wall; and the presence or absence of suspected mediastinal lymph node involvement. Other advantages of CT are its widespread availability and its relatively low cost in comparison with more advanced imaging modalities, such as PET–CT (PET combined with CT) or magnetic resonance imaging (MRI).
In the case of a solitary lesion without any apparent evidence of lymph node involvement, atelectasis, or postobstructive pneumonia, specific morphologic characteristics may help to differentiate benign disease from malignant disease ( Table 23.2 ). For this purpose, CT images should be thin slice, with contiguous 1-mm slices made through nodules. Lesions that are larger than 3 cm and that are located in the upper lobe are more likely to be malignant. Spiculated, lobulated, and ragged margins as well as notches and concavity in the margins are highly predictive of lung cancer, whereas smooth borders usually suggest a benign lesion. However, as many as one-third of lesions with smooth borders are malignant. A ground-glass attenuation surrounding a nodule may signify hemorrhagic infarction and is known as the so-called CT halo sign. This finding has been associated with aspergillosis, Kaposi sarcoma, granulomatosis with polyangiitis, and metastatic angiosarcoma. Adenocarcinoma in situ (previously known as bronchioalveolar carcinoma) also can produce a halo as a result of its lepidic growth. Tentacle or polygonal margins occur in association with fibrosis, alveolar infiltration, and collapsed alveoli. Regarding calcifications, laminated or concentric calcifications with a dense central core, diffuse and solid calcifications, or popcorn calcifications suggest a benign lesion. Although there is no specific pattern associated with malignancy, punctate and eccentric calcifications may be associated with lung cancer. Cavitation can occur in association with malignant nodules (most commonly squamous cell carcinoma) as well as benign diseases, including abscesses, infectious granulomas, vasculitides, early Langerhans cell histiocytosis, and pulmonary infarction. A cavity wall thickness of less than 5 mm is suggestive of a benign etiology, whereas irregular walls and a wall thickness of more than 15 mm are usually (although not always) associated with malignant lesions.
| Morphologic Characteristics | Malignant Disease | Benign Disease |
|---|---|---|
| Size | >3 cm | ≤3 cm |
| Margins | Spiculated, lobulated, ragged, notches and concavity, halo (adenocarcinoma in situ, Kaposi sarcoma, angiosarcoma), rarely smooth (up to a third of cases) | Smooth, halo (aspergillosis, granulomatosis with polyangiitis), polygonal, rarely spiculated (lipoid pneumonia, focal atelectasis, tuberculoma, and progressive massive fibrosis) |
| Calcifications/attenuations | Punctate, eccentric | Laminated and concentric, dense central core, diffuse and solid, popcorn (hamartoma) |
| Cavitations | Irregular and thicker walls (>15 mm) | Cavity wall thickness <5 mm (abscesses, infectious granulomas, vasculitides, early Langerhans cell histiocytosis, pulmonary infarction) |
| Ground glass | Subsolid GGNs (atypical adenomatous hyperplasia, adenocarcinomas in situ, minimally invasive adenocarcinomas and lepidic-predominant adenocarcinomas) | Pure GGNs |
| Growth rate | Doubling time, 20 to 400 days | Doubling time, <20 days (infectious process) or >400 days |
Lung nodules can be classified as solid or subsolid. Subsolid nodules can be part solid and part ground glass or can be pure ground-glass nodules (GGNs), defined as focal increased lung attenuation through which normal parenchymal structures such as airways, vessels, and interlobular septa are still visible. GGNs are frequently multiple, and the approach to these nodules is beyond the scope of this chapter. According to the International Association for the Study of Lung Cancer, the American Thoracic Society, and the European Respiratory Society, the presence of solid components in the GGN is associated with more invasive pathologic features, as subsolid nodules frequently represent the histologic spectrum of adenocarcinomas, including atypical adenomatous hyperplasia, adenocarcinomas in situ, minimally invasive adenocarcinomas, and lepidic-predominant adenocarcinomas. However, because of their slow growth rate and low metabolic activity, the differential diagnosis between benign and malignant subsolid lesions remains quite problematic. In fact, another relevant factor to take into account, which requires the availability of previous CT images, is the growth rate. Malignant nodules have a volume-doubling time of 20 days to 400 days, although the majority of cancers double in volume within 100 days. A doubling time of more than 400 days is usually associated with benign disease, whereas a doubling time of less than 20 days indicates very rapid growth and strongly suggests infectious processes.
The presence of features suggestive of intrathoracic invasion is an important clue that helps to distinguish between benign and malignant nodules. Although numerous criteria have been used to define lymph node involvement, the most widely used criterion is a short-axis diameter of more than 1 cm on a transverse CT image. In a systematic review of the medical literature relating to the accuracy of CT staging of the mediastinum, the median sensitivity and specificity of CT for identifying mediastinal lymph node metastasis were 55% and 81%, respectively. Although these studies were statistically heterogeneous, the findings are similar to the results of meta-analyses addressing the accuracy of CT for staging of the mediastinum in NSCLC, which have demonstrated very low sensitivity, ranging from 51% to 64%. In fact, as many as 20% of patients who have T1 N0 M0 disease diagnosed on the basis of CT imaging are still found to have positive lymph node involvement on the basis of surgical lymph node sampling. Moreover, although pooled specificity values (range, 76% to 84%) are higher overall than the sensitivity values, a consistent rate of lymph nodes that are defined as malignant on the basis of CT is actually benign.
The increasing availability of 18 F-2-deoxy- d -glucose ( 18 FDG)-PET in clinical practice has allowed the technique to become an essential tool for the diagnosis and staging of disease in patients with radiographic and clinical findings consistent with lung cancer. 18 FDG-PET is very accurate for differentiating benign from malignant lesions with nodules as small as 1 cm and may also detect clinically unexpected distant metastasis. Increased cellular uptake, defined as a standardized uptake value of more than 2.5, is a common property of both neoplastic and inflamed tissues. However, the sensitivity for lesions smaller than 1 cm is quiet low, likely due to lower metabolic activity, well-differentiated low-grade malignancies not being detected, and a high false-positive rate from inflammation. For mediastinal staging, PET has been shown to have both higher sensitivity and specificity than CT. A meta-analysis of data on PET demonstrated pooled estimates of 74% (95% confidence interval [CI], 69% to 79%) and 85% (95% CI, 82% to 88%) for sensitivity and specificity, respectively. However, in the context of a cancer that is still confined to the chest and thus is potentially curable, the rate of false-positive findings, which may result in missed opportunities for surgical resection, is too high. Moreover, some studies have shown a direct correlation between the accuracy of PET and increasing lymph node size on CT, where the sensitivity is higher (but specificity is lower) when nodes are enlarged.
Newer-generation integrated PET–CT imagers have combined the advantages of both modalities, allowing correlation between CT (which demonstrates anatomic details) and 18 FDG-PET (which identifies aspects of tumor function and metabolism). For differentiating between malignant and benign lung nodules, PET–CT is associated with a significantly higher specificity than CT or PET alone because of the ability to discard false-positive uptake on PETs on the basis of the morphologic findings on CT.
Lastly, dynamic MRI is an emerging diagnostic tool for the differential diagnosis and staging of lung cancer. One advantage of this tool is that it does not involve the use of ionizing radiation. Available data suggest that MRI is at least as accurate as CT in evaluating the mediastinum, and because MRI can detect differences in intensity between normal tissues and tumor, it may be superior in the ability to detect tumor invasion into the mediastinum, chest wall, diaphragm, or vertebral bodies. In fact, MRI excels in the delineation of superior sulcus tumors, including tumors that involve the neural foramina, spinal canal, and brachial plexus. ACCP and NICE guidelines currently state that MRI should not routinely be performed to assess the stage of the primary tumor, but that MRI is useful for patients with superior sulcus tumors.
Overall, the available data indicate that when the findings of noninvasive imaging (combined PET–CT, PET or CT alone, and MRI) suggest malignancy, it represents an excellent guide to determine the proper technique to achieve the final diagnosis and staging. However, especially in the context of a suspected lung cancer that is confined to the chest and a high baseline suspicion of disease, indeterminate nodules or negative mediastinal findings require additional procedures to obtain a tissue diagnosis.
Diagnostic Approach for Establishing a Definitive Diagnosis and Staging
As previously stated, the method for definitive diagnosis and staging of lung cancer depends on the suspected cell subtype (SCLC or NSCLC), the size and location of the primary tumor, the presence or absence of radiographic findings suggestive of mediastinal involvement, and the overall clinical status of the patient.
The ACCP guidelines recommend that, for patients in whom SCLC is suspected on the basis of radiographic and clinical findings, the diagnosis should be confirmed with whatever method is easiest (sputum cytology, thoracentesis, bronchoscopy, or transthoracic fine-needle aspiration). For patients in whom NSCLC is suspected, the method of achieving a diagnosis is usually dictated by the presumptive stage of the disease, as the main goal is to maximize the yield of the selected procedure by establishing both diagnosis and staging with one test and avoiding unnecessary invasive tests. The NICE guidelines also recommend choosing investigations that give the most information about diagnosis and staging with the least risk to the patient. After distant metastases have been ruled out, lung cancer presentation can be separated into four categories with respect to intrathoracic radiographic characteristics: (1) extensive mediastinal infiltration by tumor, (2) enlarged discrete N2 or N3 nodes, (3) central tumor or a tumor with enlarged N1 nodes but a normal mediastinum, and (4) peripheral small tumor with normal-sized lymph nodes. For patients who have extensive infiltration of the mediastinum, defined as a mass that infiltrates and encircles the vessels and airways such that mediastinal lymph nodes are no longer visible, the diagnosis of lung cancer should be established with the least-invasive and safest method. In cases in which mediastinal involvement (stage III) is suspected, sampling the mediastinum rather than the primary tumor offers the advantage of diagnosis and staging in one procedure.
In patients with discrete mediastinal lymph node enlargement on CT or PET, mediastinal involvement must be confirmed, and, for that purpose, endoscopic techniques such as endobronchial ultrasound (EBUS) or endoscopic ultrasound (EUS)-guided sampling are preferred as a first step over surgical staging, as they are less invasive and less costly than mediastinoscopy. However, in cases in which the results of EBUS or EUS are negative, mediastinoscopy is recommended. The absence of findings suggestive of mediastinal involvement on both CT and PET images has a high negative predictive value, except in the case of a centrally located tumor or N1 lymph node enlargement. These factors make the chance of N2 or N3 involvement relatively high (20% to 25%) and the use of a technique such as EBUS, EUS, or combined EBUS and EUS-guided needle aspiration is suggested to confirm the staging. Conversely, in the presence of peripheral lung nodules, the chance of mediastinal involvement is quite low.
Different techniques are available to achieve the diagnosis of the primary tumor, and the choice among them is largely guided by the size and location of the lesion. Sputum cytology is particularly useful for the evaluation of patients who have a centrally located tumor and patients who have hemoptysis. However, if sputum cytology is negative for carcinoma, additional testing is recommended.
For patients with suspected lung cancer who have a pleural effusion, thoracentesis is recommended to diagnose the cause of the effusion. If cytologic examination of pleural fluid is negative, image-guided pleural biopsy or medical or surgical thoracoscopy is recommended. However, if CT of the chest shows pleural thickening or pleural nodules and/or masses, image-guided needle biopsy may be considered as a first approach.
Endoscopic Techniques for Diagnosis and Staging
Over the past decade, endoscopic techniques (bronchoscopy and esophageal endoscopy) have emerged as procedures of choice for the diagnosis and staging of lung cancer. With the addition of real-time ultrasound guidance, endoscopic procedures have demonstrated accuracy for mediastinal staging, with pooled sensitivities comparing favorably with that of the traditional criterion standard, mediastinoscopy. In addition, endoscopic techniques are associated with lower morbidity and mortality and are more cost effective than mediastinoscopy.
Flexible Bronchoscopy
Flexible bronchoscopy should be offered to every patient with central lesions on CT for whom nodal staging does not influence treatment. In addition, patients with suspected lung cancer may have symptoms due to endobronchial involvement that require airway inspection with bronchoscopy for tissue sampling in order to make a diagnosis or to guide further interventions. Endobronchial biopsies provide the highest sensitivity (74%), followed by brushings (61%), and washings (47%). A combination of these methods provides a diagnosis in 88% of cases. Endobronchial needle aspiration may provide deeper penetration with less hemorrhage, and the use of this method in addition to forceps biopsies and brushings may improve sensitivity to 95%.
The sensitivity of bronchoscopy for peripheral nodules is lower than for central lesions. Transbronchial needle aspiration (TBNA) and transbronchial biopsy have provided the highest sensitivity, followed by transbronchial brushings and lavage or washing. However, the overall diagnostic accuracy largely depends on the size of the suspected primary tumor, as it has been reported to be 34% for lesions smaller than 2 cm. Therefore for patients with suspected peripheral lung nodules with an indeterminate likelihood of malignancy, for whom a tissue diagnosis is required before surgical resection, appropriate techniques of sampling include fluoroscopic guidance, radial EBUS, electromagnetic navigation bronchoscopy, and transthoracic needle aspiration (TTNA). When the lesion is moderately to highly suspicious for lung cancer, an upfront surgical excision performed via thoracoscopy is the most definitive method for establishing a diagnosis.
EBUS Fine-Needle Aspiration
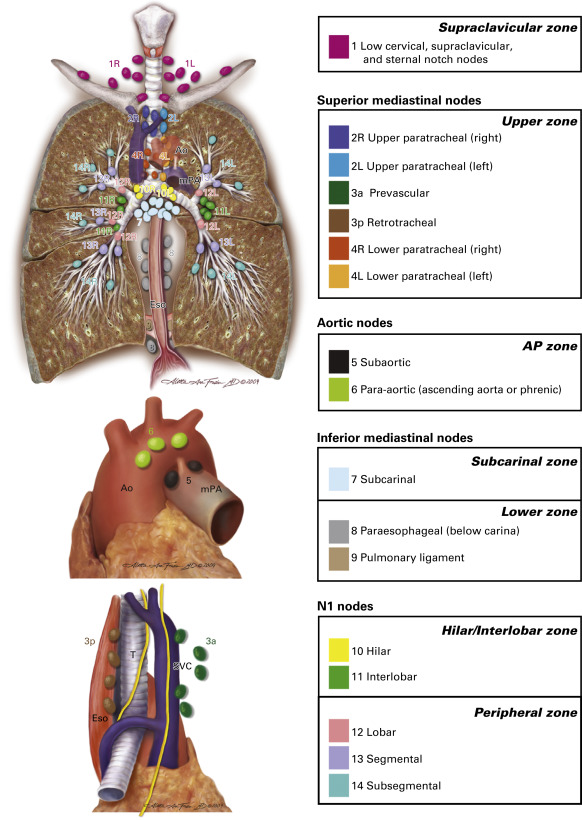
Although still associated with high diagnostic yield in expert hands, TBNA is increasingly being supplanted by EBUS fine-needle aspiration. At best, TBNA can be used for selective mediastinal staging, as only subcarinal and right paratracheal lymph nodes are reliably sampled by most bronchoscopists. Because of its limitations, TBNA has a low pooled sensitivity of 39% (95% CI, 17% to 61%), as reported by Holty et al. in a meta-analysis of 13 studies. The use of real-time ultrasound to directly visualize the target during EBUS fine-needle aspiration has solved major deficiencies associated with traditional TBNA. EBUS–TBNA is a versatile and accurate tool for the simultaneous diagnosis and staging of mediastinal and hilar lymph nodes or masses. Its range includes the highest mediastinal lymph nodes (station 1), upper paratracheal lymph nodes (stations 2R and 2L), lower paratracheal lymph nodes (stations 4R and 4L), subcarinal lymph nodes (station 7), hilar lymph nodes (station 10), and interlobar lymph nodes (station 11). From a technical standpoint, para-aortic lymph nodes (station 6) and aortopulmonary window or subaortic lymph nodes (station 5) typically require a surgical approach, whereas paraesophageal lymph nodes (station 8) and pulmonary ligament lymph nodes (station 9) are usually best accessed with use of EUS fine-needle aspiration ( Fig. 23.1 ).