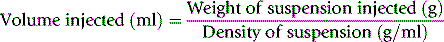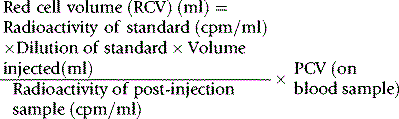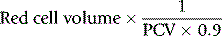Chapter 17 Diagnostic radioisotopes in haematology
Other investigations that may have haematological interest are more likely to be referred to a department of medical physics or nuclear medicine. Even when the tests are not carried out directly in the haematology department, it is essential for the haematologist to understand their principles and limitations and to be able to interpret the results in clinical terms. Various textbooks1,2 provide more complete accounts of the theory and practice of nuclear medicine techniques, as does a monograph on radioisotopes in haematology by Lewis and Bayly.3
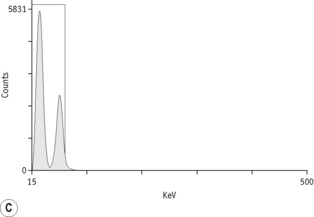
The main properties of the radioisotopes useful in diagnostic haematology are shown in Table 17.1. The units used to express radioactivity and the effects of radiation on the body are given in the previous edition. Anyone handling radioisotopes must be aware of the potential radiation hazard. It is also important to be aware of the potential biohazard of handling blood products and administering them to patients (see Chapter 24).
Radiation protection
The quantity of radioactivity used in diagnostic work is usually small and good laboratory practice is all that is necessary for safe working. However, before using radioisotopes, workers should be familiar with the regulations concerning radiation protection for themselves, their fellow workers and patients.4
The effect of radiation on the body depends on the amount of energy deposited and is expressed in grays (Gy). The unit that describes the overall effect of radiation on the body, or the ‘effective dose,’ is measured in sieverts (Sv) or millisieverts (mSv). The annual whole-body dose limit for somebody working with radioisotopes is in the order of 20 mSv, whereas 1 mSv is the annual limit for the general public. To put this into perspective, 1 mSv is produced by normal background radiation in about 6 months and the radiation dose from a single chest X-ray is 0.02 mSv.5 No statutory limit of total annual radiation dose has been set for patients, but it is an important requirement that radioisotopes should be handled only in approved laboratories under the direction of a trained person who holds a certificate from the appropriate authority specifying the radioisotopes that the individual is authorized to use and the dose limits that must not be exceeded. In the UK, this authority is the Administration of Radioactive Substances Advisory Committee (ARSAC).5 Radioisotopes should not be given to pregnant women unless the investigation is considered imperative; if an investigation is necessary during lactation, breast-feeding should be discontinued until radioactivity is no longer detectable in the milk. When radioisotope investigations are necessary in children, the dose relative to that for an adult should be based on body weight (Table 17.2).
Table 17.2 Radioisotope doses for children as a decimal fraction of the adult dose
| Weight (kg) | Fraction of adult dose |
|---|---|
| 10 | 0.3 |
| 15 | 0.4 |
| 20 | 0.5 |
| 30 | 0.6 |
| 40 | 0.75 |
| 50 | 0.9 |
| 60 | 0.95 |
| 70 | 1.0 |
The laboratory (premises) using radioisotopes should be registered to store, handle and dispose of radioactive materials, and appropriate permits are obtained under the Environmental Permitting Regulations 2010.5a
Protective gloves must always be worn when handling radioisotopes; any activity that does get on the hands can usually be removed by washing with soap and water or, if that fails, with a detergent solution. For each laboratory in which isotopes are used, a radiation protection supervisor (RPS) should be nominated to supervise protection procedures and to ensure that a careful record is kept of all administered radioisotopes. This RPS should work in association with the departmental safety officer (see p. 579) and must ensure that all personnel working with radioactive materials wear dosimetry badges (available from an approved dosimetry service provider, e.g. Landauer, Oxford; ISO Pharma, Norway), which must be checked at regular intervals.
Apparatus for Measuring Radioactivity in Vivo
Imaging
The most widely used method for imaging is by the scintillation camera (gamma camera). It consists of a lead shielding, a large thin sodium iodide detector, an array of photomultiplier tubes, a collimator with multiple parallel holes and a system for pulse height analysis and for storage and display of the data. By scanning down the body, an image of the distribution of the label is built up and recorded. It can also be used to measure the quantity of the isotope in various organs. By rotating the scintillation camera around the body, single-photon emission computed tomography (SPECT) can be performed to produce sectional images. Positron emission tomography (PET) has augmented scintillation scanning and uses radioisotopes that are positron emitters.6,7
Measurement of Radioactivity with a Scintillation Counter
Standardization of Working Conditions
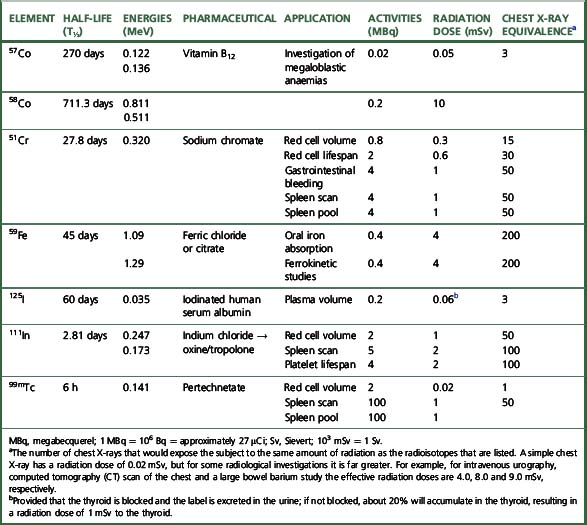
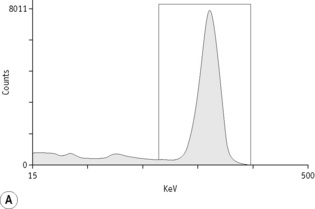
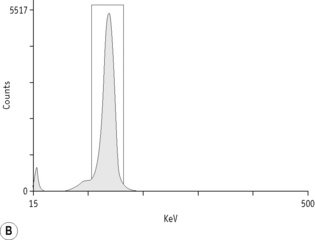
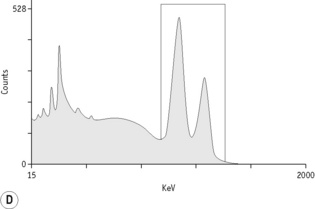
For each radioisotope, it is necessary to plot a spectrum of pulse height distribution and to identify a window corresponding to the energy at which the maximum number of pulses is emitted. Examples of spectra and selected settings are illustrated in Figure 17.1. The setting of the apparatus, once determined, should remain constant for many months.
Counting Technique
Measurement of radioactivity
Measurements are usually carried out for a fixed time period and the results are recorded as counts per second (cps) or counts per minute (cpm). Radioactivity is subject to random but statistically predictable variation similar to that in blood cell counts (see p. 612). The accuracy of the count depends on the total number of the counts recorded as the variance (σ) of a radioactive count = √ total count.
Correction for Physical Decay
Because physical decay is a continuous process that proceeds at an exponential rate, it is possible to correct mathematically for the loss of radioactivity and to convert any measurement back to the initial reference time. This is necessary when comparing successive observations made at different times after the administration of a radionuclide to a patient.8
Blood volume
The haemoglobin concentration (Hb), red cell count and packed cell volume or haematocrit (PCV/Hct) do not invariably reflect the total red cell volume (RCV). Whereas in most cases for practical purposes, there is adequate correlation between peripheral blood values and (total) RCV,8 there will be a discrepancy if the plasma volume is reduced or increased disproportionately. Fluctuation in plasma volume may result in haemodilution, giving rise to pseudoanaemia, or conversely, haemoconcentration, giving rise to pseudopolycythaemia.
Measurement of Blood Volume
Principle
In contrast to measurement of RCV, plasma volume measurements are only approximations because the labelled albumin undergoes continuous slow interchange between the plasma and extravascular fluids, even during the mixing period. For this reason, it is undesirable to attempt to calculate RCV from plasma volume on the basis of the observed PCV. However, because the RCV is generally more stable, calculation of TBV from RCV is usually more reliable, provided that the difference between whole-body and venous PCV is appreciated and allowed for (see p. 379). Measurement of red cell and plasma volumes separately by direct methods is to be preferred.
Red Cell Volume
Radioactive Chromium Method
For the radioactive chromium method,9 add approximately 10 ml of blood to 1.5 ml of sterile National Institutes of Health (NIH)-A acid–citrate–dextrose (ACD) solution (see p. 619) in a sterile bottle with a screw cap. Centrifuge at 1200–1500 g for 5 min. Discard the supernatant plasma and buffy coat and slowly, with continuous mixing, add to the cells 8 × 103 Bq of Na251CrO4 per kg of body weight. The sodium chromate should be in a volume of at least 0.2 ml, being diluted in 9 g/l NaCl (saline). Allow the blood to stand for 15 min at 37°C for labelling to take place. Wash the red cells twice in 4–5 volumes of sterile saline: for all procedures requiring sterile saline, this should be 9 g/l (0.9%) sodium chloride BP (non-pyrogenic); 12 g/l NaCl should be used when red cell osmotic fragility is greatly increased (e.g. in cases of hereditary spherocytosis).
Measure the PCV of each sample. PCV should be obtained by microhaematocrit centrifugation for 5 min or for 10 min if the PCV is more than 0.50 and correcting for trapped plasma by deducting 2% from the measurement. A more accurate measurement of the PCV can be obtained by the International Council for Standardization in Haematology (ICSH) surrogate reference method (see p. 30).
Technetium Method
Indium is available as 111In chloride. The labelling procedure is simpler than with 99mTc and, because there is less elution than with technetium during the first hour,10 it is particularly suitable for delayed sampling. For labelling blood cells, the indium is complexed with oxine11 or tropolone.12
Plasma Volume
Calculating Total Blood Volume
Whole-body and venous packed cell volume ratio
PCV measured on venous blood is not identical to the average PCV of all the blood in the body. This is mainly because the red cell:plasma ratio is less in small blood vessels (capillaries, arterioles and venules) than in large vessels. The ratio between the whole-body PCV and venous blood PCV is normally about 0.9,9 and it is thus necessary in the calculation of TBV from measurements of RCV to multiply the observed PCV by 0.9. Thus, TBV is given by the following: