Keywords: Diagnosis; lung cancer; mind mapping; molecular; staging
Summary of Key Points
- •
Mind maps are used as a tool to illustrate the principles involved in the diagnostic evaluation of a patient with known or suspected lung cancer.
- •
Addressed are factors important in establishing the diagnosis and defining the extent of disease; the histologic and molecular classifications of lung cancer; and the potential role for repeat biopsy in patients with acquired resistance to targeted therapies.
- •
Reviewed are diagnostic platforms for tumor profiling in the clinical setting and the use of these technologies in the noninvasive evaluation of surrogate tissues for both disease monitoring and early diagnosis.
The personalized treatment of lung cancer begins with an accurate diagnosis and assessment of both the extent of disease and other clinically relevant prognostic and predictive factors that are necessary to define the optimal treatment approach. Treatment decision making relies on a number of both patient- and tumor-specific factors. Lung cancer is a heterogeneous disease, and recent advances in molecular analysis and the development of targeted therapy approaches have added to the complexity of the diagnostic evaluation. In addition, revisions in the staging system, changes in the pathologic classification, and the addition of minimally invasive diagnostic modalities have increased the importance of a coordinated multidisciplinary team approach to establish both the diagnosis and the tumor stage as efficiently as possible.
Mind mapping was developed as a technique to visually represent ideas and their nonlinear relationships. Mind maps have been used as teaching tools in a number of different fields, including medical education, to present complex information and improve recall. Originally developed by Tony Buzan, a mind map starts with a central idea or key concept, which is then linked by branches to related ideas. Mind maps include color and pictures to illustrate the intra- and inter-relationship between ideas. Unlike a linear algorithm or a concept map, a mind map begins with a central idea, and its relationships are depicted radially (i.e., radial or spider map). The information is organized hierarchically, with the most general information at the center and more detailed information depicted at the extremes of each relationship branch.
In this appendix, we use mind maps as a tool to illustrate the principles involved in the diagnostic evaluation of a patient with known or suspected lung cancer. The initial mind maps address the factors that are important in establishing the diagnosis and defining the extent of disease. Because of the growing importance of targeted therapies, we use this technique to illustrate both the histologic and molecular classifications of lung cancer. In addition, we address the potential role for repeat biopsy in patients with acquired resistance to targeted therapies. Lastly, we use mind maps to review diagnostic platforms for tumor profiling in the clinical setting and to assess the potential to use these technologies in the noninvasive evaluation of surrogate tissues for both disease monitoring and early diagnosis.
Initial Evaluation
History and Physical
Fig. A.1 includes a mind map that outlines the factors that are important to address during the initial history and physical examination of a patient suspected of having lung cancer. The objectives of the initial evaluation are to estimate the probability that lung cancer is present and to assess for evidence of distant metastatic disease. In addition, the initial evaluation should clarify patient-specific factors that may affect treatment decision making if cancer is confirmed, including comorbidities, performance status, underlying lung function, and the patient’s values and goals.
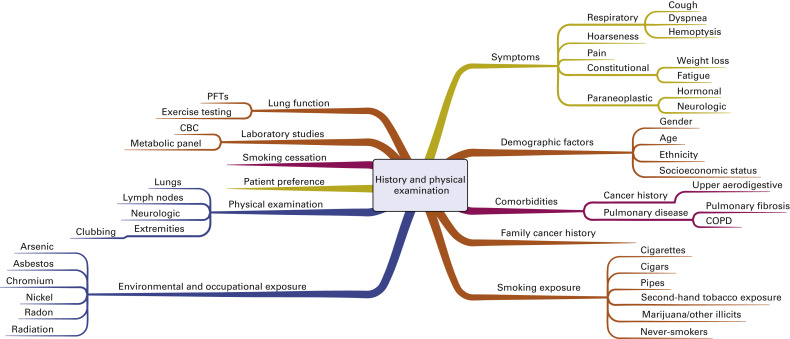
The initial risk assessment for lung cancer includes an evaluation of symptoms. Most patients with lung cancer will have symptoms at presentation. Symptoms may be due to the primary tumor or due to regional or distant spread of disease. Constitutional symptoms, including weight loss or fatigue, are important to consider because they may reflect an advanced stage of lung cancer. Several paraneoplastic syndromes have also been described, each with potentially unique clinical manifestations. Early recognition of a paraneoplastic process is important to minimize the risk for long-term morbidity and mortality.
The most common risk factor for lung cancer remains tobacco smoking. Lung cancer is 10 to 20 times more likely to develop in individuals with a history of smoking than in persons who have never smoked. The individual risk is affected by age as well as the duration and intensity of smoking. Lung cancer develops in only a minority of smokers, however, so inherited factors may also play a role in lung cancer risk. Other environmental and occupational exposures are also important to consider (see Fig. A.1 ). For example, pollution is increasingly being recognized as a risk factor for lung cancer. Important personal risk factors include age, gender, ethnicity, socioeconomic status, and comorbidities, including acquired lung disease. Although more men than women will die of lung cancer, the gender gap is narrowing, and lung cancer is the leading cause of cancer-related mortality among women in the United States. Although the rates of lung cancer are similar for black and white women, the incidence of lung cancer is higher among black men than among white men. Both the incidence of and mortality from lung cancer are higher in populations of lower socioeconomic status.
Radiographic Evaluation
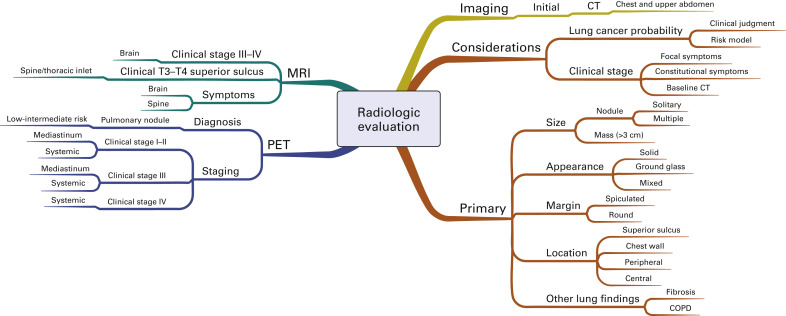
Fig. A.2 is a mind map that illustrates the radiologic evaluation. All patients should have initial computed tomography (CT) of the chest and upper abdomen that extends through the adrenal glands. The decision to pursue additional imaging for diagnosis and staging depends on both the estimated risk of lung cancer and the initial clinical stage. When patients have focal symptoms suggestive of metastatic disease, directed imaging (e.g., magnetic resonance imaging of the spine or brain) is recommended for confirmation. The radiographic characteristics of the primary abnormality in the lung that affect the risk assessment include the size, radiographic appearance, margin, and location of this lesion, as well as any underlying lung disease or additional lesions. Positron emission tomography (PET) is used in the diagnostic evaluation to characterize pulmonary nodules considered to be at low to intermediate risk for lung cancer. PET is not recommended to characterize abnormalities considered to be at high risk for lung cancer. However, PET is frequently recommended for mediastinal and systemic staging in most patients, including patients with early (stage I–II), locally advanced (stage IIIA–IIIB), and metastatic (stage IV) disease. In addition to PET, magnetic resonance imaging of the brain is frequently recommended for staging of patients with clinical stage III or IV disease.
Invasive Diagnosis
Options for tissue confirmation of the lung cancer diagnosis are outlined in Fig. A.3 . Important considerations before pursuing biopsy include the probability of lung cancer and the estimated clinical stage, as well as the feasibility, risk, diagnostic yield, and the potential to obtain adequate tissue for both histologic confirmation and molecular analysis. Patient-specific factors outlined previously are also important to consider, including performance status, comorbidities, pulmonary function, and the patient’s values and goals. Given the complexity of treatment decision making, multidisciplinary tumor boards are generally recommended, if available, to help coordinate the diagnostic plan.
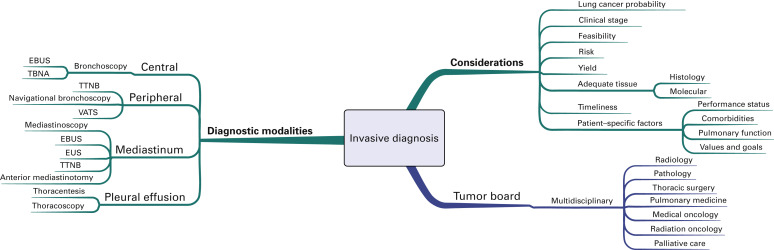
The most important principle is to take a biopsy specimen from the most distant site of disease to confirm both diagnosis and stage. If obtaining this type of specimen is not considered feasible or safe, but the patient has a high likelihood of distant metastatic disease on the basis of clinical presentation and imaging, then a biopsy specimen of the safest site is generally recommended. Solitary abnormalities at a distant metastatic site evident on PET images warrant tissue confirmation because false-positive imaging will affect both treatment planning and intent. Likewise, clinical stage III disease should be confirmed by pathologic analysis. Mediastinoscopy remains the criterion standard for pathologic confirmation of N2 and N3 disease. However, image-guided needle techniques, including endobronchial ultrasound-guided transbronchial needle aspiration and endoscopic ultrasound-guided biopsy, are increasingly being recognized as valid alternatives to mediastinoscopy for mediastinal staging.
If a patient is considered to have an early stage (I–II) lung cancer, a thoracic surgery evaluation should be pursued before a needle biopsy is performed for tissue confirmation because resection could be used as the primary means of both diagnosis and treatment. If that patient is not considered a surgical candidate because of comorbidities, poor underlying lung function, or unwillingness to have an operation, then tissue confirmation should be pursued. The specific procedure will depend on tumor location as well as the other factors indicated earlier (see Fig. A.3 ).
Pathologic Classification of Lung Cancer
Tumor Histology
In 2011, the International Association for the Study of Lung Cancer, the American Thoracic Society, and the European Respiratory Society published a new pathologic classification system for lung cancer. This new system is separated into two components by the method in which the tissue was obtained; standardized diagnostic criteria are provided for small biopsy specimens and cytologic specimens as well as for resected specimens. A mind map shows this new classification system ( Fig. A.4 ). Most patients will have advanced disease at presentation, and the diagnosis will be confirmed by evaluation of a small biopsy specimen. Given the therapeutic implications, emphasis is placed on establishing the specific histologic subtype, including adenocarcinoma, squamous cell carcinoma, and small cell carcinoma. The previous designation of large cell neuroendocrine carcinoma is now classified as nonsmall cell lung cancer (NSCLC) with neuroendocrine morphology and positive or negative neuroendocrine markers. For those tumors without classic morphologic features, the new system includes recommendations for limited special stains to determine the subtype of carcinoma beyond NSCLC, not otherwise specified. If an adenocarcinoma marker is positive (e.g., thyroid transcription factor-1), then the tumor is classified as NSCLC-favor adenocarcinoma, and if a squamous cell marker is positive (e.g., p40), then the tumor is classified as NSCLC-favor squamous cell carcinoma.
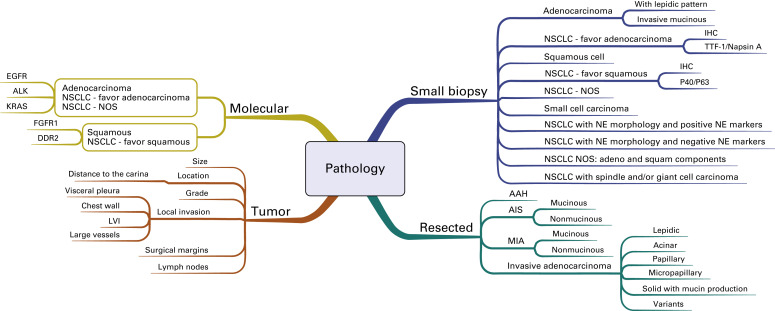
For resected specimens, the term bronchioloalveolar cell carcinoma has been discontinued, and the terms adenocarcinoma in situ and minimally invasive adenocarcinoma have been added. Both of these adenocarcinomas are defined as small tumors (3 cm or less) with a lepidic growth pattern. Minimally invasive adenocarcinoma includes tumors with no more than 5 mm of invasion. Larger tumors with more than 5 mm of invasion are designated as invasive adenocarcinoma, and the subtype is determined based on the predominant growth pattern. In validation studies, the proposed subtyping contained in this new system was shown to have prognostic significance. In addition to the histologic subtype, the reporting of resected tumors should include descriptions of the size, location, grade, margins, pleural involvement, lymphovascular invasion, and lymph node involvement by station.
Molecular Classification
This IASLC/ATS/ERS pathologic classification system also emphasizes the importance of molecular testing based on tumor histology. An institutional multidisciplinary strategy for obtaining and processing small biopsy specimens should be established to make sure that sufficient tissue is available for both histologic classification and molecular analysis. Given the clinical validation of mutations in the epidermal growth factor receptor (EGFR) and translocations of anaplastic lymphoma kinase (ALK) as therapeutic targets, current guidelines from the American Society of Clinical Oncology and the National Comprehensive Cancer Network, as well as a joint guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and the Association for Molecular Pathology, recommend testing adenocarcinoma of the lung for these two markers at diagnosis before selecting patients for directed therapy.
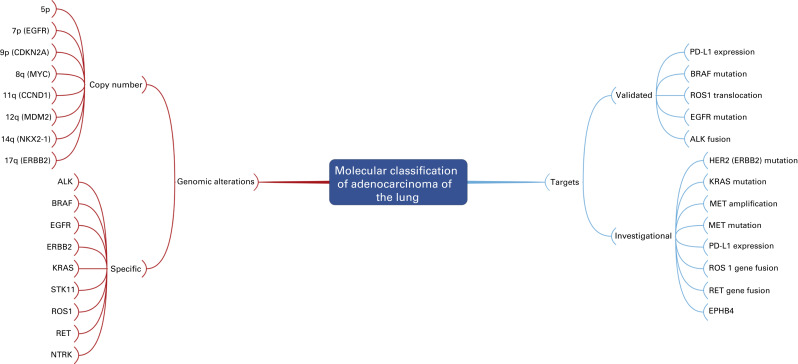
Fig. A.5 is a mind map that illustrates the molecular classification of adenocarcinoma of the lung. In addition to EGFR mutations and ALK translocations, increasing numbers of other so-called driver mutations and gene fusions have been described. Given the limited overlap, these molecular alterations define unique subsets of lung adenocarcinoma and can potentially be used to select patients with advanced adenocarcinoma of the lung for molecularly targeted therapy as well as targeted immunotherapy indicated by increased activity of PD-1/PD-L1 pathway inhibitors. Investigators from the Clinical Lung Cancer Genome Project and Network Genomic Medicine (CLCGP/NGM) characterized genome alterations in 1255 clinically annotated lung tumors. Overall, more than 55% of the tumors had at least one genomic alteration that was potentially amenable to targeted therapy. The pattern of genomic alterations differed among histologic subsets. This mind map includes an illustration of the genomic alterations that were relatively common in lung adenocarcinoma.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree