Summary of Key Points
- •
Neuroendocrine cancers of the lung share some fundamental pathology features but span a broad range of clinical behaviors.
- •
The clinical behavior varies with the degree of differentiation: typical carcinoids are more indolent and localized, atypical carcinoids are intermediate and tend to spread systemically, and large cell neuroendocrine cancers are high grade and aggressive similar to small cell cancers.
- •
Unlike midgut carcinoids, bronchial carcinoids seldom present with carcinoid syndrome.
- •
Surgical resection is the preferred treatment for early-stage disease.
- •
Standard chemotherapy treatments for metastatic disease are platinum based but little phase III evidence exists to support a specific regimen.
- •
Clinical trials of molecularly targeted therapy are urgently needed.
Pulmonary neuroendocrine tumors represent a spectrum of tumors that develop from neuroendocrine cells of the bronchopulmonary (BP) epithelium. Although neuroendocrine tumors have similar morphologic and immunohistochemical (IHC) features, they span a broad clinical–pathologic spectrum and are characterized by differing biologic behavior. Typical carcinoids are low-grade, slow-growing malignancies that rarely metastasize; atypical carcinoids are intermediate-grade malignancies; and large cell neuroendocrine carcinoma (LCNEC) and small cell lung cancers (SCLCs) are high-grade carcinomas.
BP carcinoid tumors are part of a heterogeneous group of neuroendocrine tumors arising from the Kulchitzky cells present in the bronchial mucosa and account for approximately 1% to 2% of all lung malignancies in adults. The incidence of BP carcinoid tumors in the United States has increased by approximately 6% per year over the past 30 years, with rates ranging from 0.2 to 2 per 100,000 individuals per year.
LCNEC is an uncommon tumor of the lung with an incidence of approximately 3%, as reported in surgical case series. LCNEC has only recently been described as a form of high-grade lung cancer that expresses neuroendocrine features. Because of the low incidence, evolving classification, and high degree of diagnostic difficulty, many aspects of LCNEC are still unknown.
Classification
Although neuroendocrine tumors of the lung exhibit divergent behavior, they share similar morphologic and biochemical characteristics, including an ability to secrete neuropeptides, and the presence of neuroendocrine granules. Of the pulmonary carcinoids, 80% to 90% are typical carcinoids, whereas 10% to 20% are atypical carcinoids.
Before the introduction of LCNEC, neuroendocrine tumors of the lung were subdivided into three classes: typical carcinoids, atypical carcinoids, and SCLC. In 1991, after reviewing a set of neuroendocrine tumors (typical and atypical carcinoids and SCLC), Travis et al. found a group of tumors that deviated from the known classification in prognosis and morphology. They classified this new group as a high-grade neuroendocrine nonsmall cell lung cancer (NSCLC) and placed it in between atypical carcinoid and SCLC.
In 1991, LCNEC was recognized in the World Health Organization (WHO) classification of lung tumors as a large cell carcinoma (LCC) based on its cytologic parallels (large cell size and abundant cytoplasm). LCNEC is different from other LCCs because of its combination of neuroendocrine differentiation and morphology. Other LCC histologies can express neuroendocrine morphology, but not in combination with neuroendocrine differentiation.
Other regularly used terms referring to LCNEC are neuroendocrine carcinoma (NEC) grade 3, poorly differentiated NEC, and high-grade NSCLC; however, these terms are also used for SCLC and thus may include tumors with SCLC and/or LCNEC histology.
The most recent WHO/International Association for the Study of Lung Cancer classification of neuroendocrine lung tumors was established in 2004 by Travis et al. ( Table 55.1 ).
| Neuroendocrine Tumor | Criteria for Diagnosis |
|---|---|
| Typical carcinoid | Tumor with carcinoid morphology and <2 mitoses/2 mm 2 (10 HPFs), lacking necrosis, and ≥0.5 cm |
| Atypical carcinoid | Tumor with carcinoid morphology with 2–10 mitosis/2 mm 2 (10 HPFs) OR necrosis (often punctate) |
| Large cell neuroendocrine carcinoma | Tumor with a neuroendocrine morphology (organoid nesting, palisading, rosettes, trabeculae) High mitotic rate: ≥11/2 mm 2 (10 HPFs), median of 70/2 mm 2 (10 HPFs) Necrosis (often large zones) Cytologic features of a nonsmall cell carcinoma (NSCLC): large cell size, low nuclear-to-cytoplasmic ratio, vesicular, coarse or fine chromatin, and/or frequent nucleoli. (Some tumors have fine nuclear chromatin and lack nucleoli, but qualify as NSCLC because of large cell size and abundant cytoplasm.) Positive immunohistochemical staining for one or more neuroendocrine markers (other than neuron-specific enolase) and/or neuroendocrine granules by electron microscopy |
| Small cell carcinoma | Small size (generally less than the diameter of three small resting lymphocytes) Scant cytoplasm Nuclei: finely granular nuclear chromatin, absent or faint nucleoli High mitotic rate: ≥11/2 mm 2 (10 HPFs), median of 80/2 mm 2 (10 HPFs) Frequent necrosis often in large zones |
Diagnosis
Histology
Bronchopulmonary Carcinoids
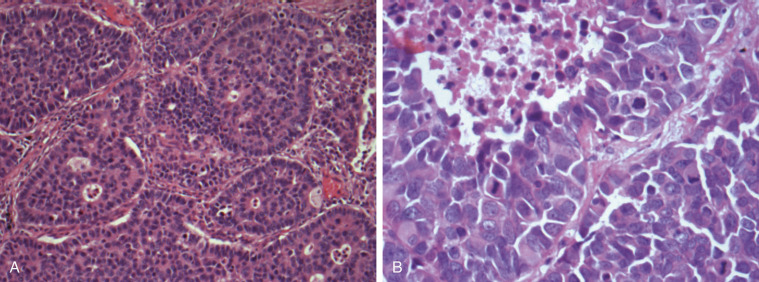
A diagnosis of BP carcinoid can be challenging because of small biopsies, crush artifacts, and poor fixation of the specimens. BP carcinoids are composed of cells containing round to oval nuclei, a moderate amount of eosinophilic cytoplasm, finely granular chromatin, and indistinct or absent nucleoli. The cells tend to be uniform and can be arranged in a variety of patterns, including trabecular, palisading, spindle cell, glandular, follicular, rosette-like, sclerosing, clear cell, and papillary. Rarely, BP carcinoids can show oncocytic or melanocytic features. Stromal hyalinization, calcification, ossification, and amyloid deposition can also occasionally be seen. Two features that distinguish atypical from typical carcinoids are the presence of necrosis and number of mitoses per square millimeter. Typical carcinoids must have no necrosis and fewer than 2 mitoses/2 mm 2 (10 high-power fields [HPFs]; Fig. 55.1 ). Atypical carcinoids should have either punctate necrosis or 2 mitoses to 10 mitoses/2 mm 2 (10 HPFs; Fig. 55.2 ), and exhibit neuroendocrine differentiation, confirmed by more than 10% of positive cells for a neuroendocrine marker, such as chromogranin, synaptophysin, and/or neural cell adhesion molecule (NCAM; CD56).
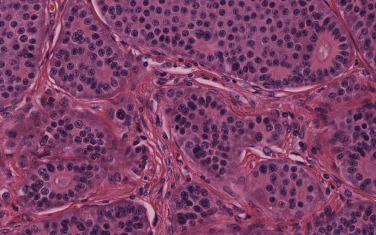
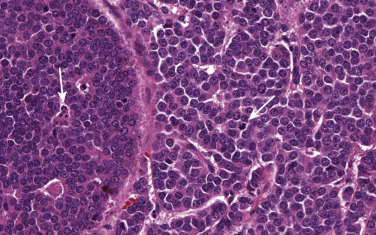
Large Cell Neuroendocrine Carcinoma
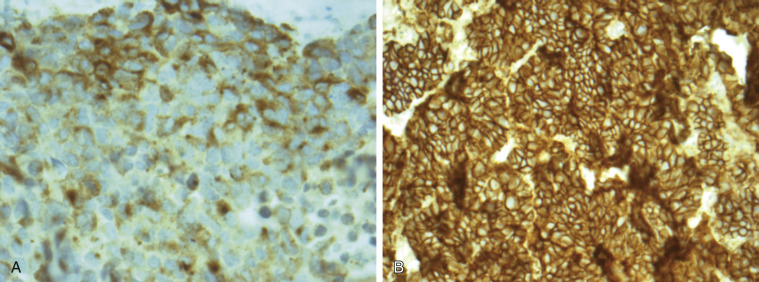
The diagnosis of LCNEC is also complex and often requires a large surgically resected lung biopsy specimen, mainly because small biopsy specimens are susceptible to crushing artifacts that may disturb the neuroendocrine morphology and cell size, two features that are critical for the diagnosis of LCNEC. To establish a LCNEC diagnosis, several histologic criteria have to be confirmed ( Table 55.1 ). LCNECs express a neuroendocrine growth pattern (morphology) that is similar to that seen in low-grade neuroendocrine tumors (carcinoids). On slides stained with hematoxylin and eosin, these neuroendocrine growth patterns are recognized as organoid nesting, trabeculae, rosette-like structures, or palisading cells ( Fig. 55.3 ). All LCNECs have a high proliferative rate, with more than 10 mitoses/mm 2 on HPF examination. Total HPF examination should include an area of 2 mm 2 , preferably in the regions with the highest mitotic activity and viable cells. Besides high mitotic activity, areas of necrosis are frequently noted. By definition, all LCNECs express neuroendocrine differentiation, which is immunohistochemically confirmed when focal activity (more than 10% positive cells) is found with use of neuroendocrine markers (chromogranin, synaptophysin, or NCAM [CD56]) ( Fig. 55.4 ). The presence of neuroendocrine granules on electron microscopy examination is also sufficient for diagnosis of LCNEC. The morphologic features of LCNEC resemble those of NSCLC, because the cells are large with abundant eosinophilic cytoplasm and a low nuclear-to-cytoplasmic ratio. The nuclei are round to oval shaped with granular chromatin (so-called salt and pepper). Nucleoli are also frequently seen ( Fig. 55.3 ).
Cytology
It can often be challenging to make a diagnosis of atypical or typical carcinoid on cytologic examination, given the limited number of cells and the absence of tissue structure. BP carcinoids present as small, polyhedral-shaped cells with oval or round nuclei. The cells are arranged in a regular fashion, consisting of nests, sheets, ribbons, or spindle structures, separated by a fibrovascular stroma; however, a spindle-cell variant exists. Necroses are only seen in atypical carcinoids, which have 2 mitoses to 10 mitoses/10 HPFs. Typical carcinoids have less than 2 mitoses/10 HPFs. Nucleoli are common in atypical carcinoids and are occasionally seen in typical carcinoids; both typical and atypical carcinoids have finely granular chromatin.
Although cytologic smears are not suitable for establishing a diagnosis of LCNEC, cytologic examination can be useful during the initial evaluation of a tumor. On cytologic smears, the presentation of LCNEC is medium-to-large round or polygonal cells arranged in groups or as a single cell. The LCNEC cells can be arranged in rosette-like structures or peripheral palisading cells, and nuclear molding can be seen. In the background of the cytologic smears, necrosis and nuclear streaking are commonly seen. LCNEC cells have scant or moderate amounts of cytoplasm with a high nuclear-to-cytoplasmic ratio, which is dependent on the fixation material (air dried vs. alcohol fixated). Most often, the nuclear shape is round or oval, and nuclear mitoses are frequently found. Nuclear pleomorphism and nucleoli are occasionally present.
Differential Diagnosis
Histology
Diagnosing LCNEC is a highly complex process and was addressed in interobserver studies on resected pulmonary neuroendocrine tumors. In a study performed by a panel of expert lung pathologists, a moderate interobserver variation with κ ranging from 0.35 to 0.81 was reported. The most common overlapping diagnoses with LCNEC were SCLC, LCC, atypical carcinoid, basaloid carcinoma, and the poorly differentiated NSCLC tumors expressing a neuroendocrine phenotype. Undoubtedly, these uncertainties are secondary to the morphologic and cytologic similarities of lung tumors with LCNEC. In the interobserver studies of BP carcinoids, there was a variability of results, from substantial agreement (κ > 0.7) in one study to fair to moderate (κ = 0.39–0.87) in another study by Lee et al. The fact that there were only two pulmonary pathologists in the Lee et al. study may have had an influence on the results.
Although differentiating pulmonary NEC from overlapping tumors can be a challenge, several criteria may help guide the diagnosis. The differentiation of atypical carcinoid from high-grade NEC can be achieved with the help of the mitosis count; on average, 2 mitoses to 10 mitoses/2 mm 2 are expressed in atypical carcinoid compared with more than 11 mitoses/2 mm 2 in high-grade NEC. Moreover, necrosis in atypical carcinoid predominantly consists of punctate foci, whereas necrosis is more prominent in LCNEC.
A distinction between LCNEC and SCLC can be established only with the help of cytology-based criteria. Compared with LCNEC, SCLC has a smaller cell size (less than the diameter of three lymphocytes), a higher nuclear:cytoplasmic ratio, and absent or faint nucleoli. Although it was demonstrated in several studies that LCNEC and SCLC had a considerably different mean cell size, the standard deviations did overlap. Therefore whether cell size is the most adequate criteria to differentiate LCNEC from SCLC should be questioned. The mitosis count, which is useful in atypical carcinoid, is not helpful in differentiating LCNEC from SCLC.
Both SCLC and typical carcinoid cells may be similar in size and shape and may appear uniform. Variation in size may be a helpful distinguishing feature, from less than onefold (mild) in typical carcinoid to more than twofold to threefold in atypical carcinoid and high-grade neuroendocrine tumors. In addition, SCLC cells are arranged in more cohesive groups and three-dimensional clusters, which are not present in bronchial carcinoids. Ki-67 (MIB-1) staining may also be useful because SCLC will show a proliferation index of more than 50%, whereas carcinoids have a proliferation index of less than 20%.
In addition to atypical carcinoid and SCLC, other forms of nonsmall carcinomas—such as the basaloid carcinoma—must be distinguished from LCNEC. The morphology of basaloid carcinoma is comparable to that of LCNEC; differentiation is possible with the help of IHC with neuroendocrine markers, which are negative in basaloid carcinomas.
As for the basaloid carcinoma, poorly differentiated adenocarcinomas or squamous cell carcinomas can also be distinguished from LCNEC with the help of IHC markers, which are addressed later in the chapter.
Cytology
The value of cytology in the diagnosis of BP carcinoids has been examined in several studies. The main factor in distinguishing typical carcinoid from high-grade neuroendocrine neoplasms or other carcinomas is adequate evaluation of nuclear features. Typical carcinoids tend to have fine, evenly dispersed granular chromatin with inconspicuous or undetectable nucleoli; SCLC has coarsely granular chromatin and occasional chromatin clumping, and poorly differentiated carcinoma has more clumped chromatin in a vesicular nucleus. The cytologic diagnosis of atypical carcinoid can be quite difficult, as it has features that may be found in typical carcinoid or SCLC. In atypical carcinoid, tumor cells tend to be larger than those noted in typical carcinoid or SCLC, and nuclear pleomorphism and atypia are also common. The finely granular chromatin is present, and mitoses range from 2 HPF to 10/10 HPFs.
Compared with histology, misdiagnoses are even more common when LCNEC is diagnosed on cytology specimens. Preoperatively obtained cytologic smears from confirmed resected LCNEC have been reviewed in several studies. In about 80% of smears, the cytology was initially diagnosed as NSCLC, SCLC, LCC with neuroendocrine features, poorly differentiated adenocarcinoma or squamous cell carcinoma, or combined SCLC. However, during the period between 1990 and the beginning of 2000, when the majority of the series in these studies were diagnosed, LCNEC was a new entity and pathologists may have been unaware of it. In 2005, a study was published in which 9 (90%) of 11 LCNEC cases ( N = 11) were correctly diagnosed before surgery.
Cytokeratin Markers
High-molecular-weight cytokeratin (CK) types 1, 5, 10, and 14 (antibody clone 34E1β2) are almost solely expressed in non-NECs such as basaloid carcinoma and poorly differentiated adenocarcinoma or squamous cell carcinoma. In tissue microarray panel studies, 3% to 17% of pure LCNEC showed positive staining for clone 34E1β2. Other commonly used high-molecular-weight CK types such as CK5/6, CK7, and CK20 were positive in 2% to 13%, 57% to 77%, and 2% to 10% of LCNECs, respectively. CK18 and CK19 were positive in 97% and 59%, respectively. In combined LCNEC, expression of 34E1β2 has been noted in the adenocarcinoma/squamous cell carcinoma component.
Markers for Differentiating Atypical From Typical Carcinoid
Counting mitoses may be challenging, especially if there is crush artifact or poor sample fixation. Ki-67 expression may be used as a surrogate to help differentiate between atypical and typical carcinoids. In a study by Warth et al., a low interobserver agreement was demonstrated when using mitotic count (median κ = 0.213) to distinguish between atypical and typical carcinoids, and the overall κ for Ki-67 was higher (0.746). Ki-67 expression is also prognostic; carcinoids with Ki-67 expression higher than 5% were associated with a worse overall survival.
Markers for Differentiating Large Cell Neuroendocrine Carcinoma From Squamous Cell Carcinoma
In addition to high-molecular-weight CKs, markers such as desmocollin-3 and p63 may be useful in discriminating poorly differentiated squamous cell carcinomas from LCNEC. Desmocollin-3 was negative in 99% of all pure LCNECs and therefore appears to be a promising specific marker but requires further validation. Expression of p63, a highly sensitive and specific marker for diagnosing squamous cell carcinomas, was detected in 0% to 18% of LCNECs. Therefore p63 may not be appropriate as a specific marker for poorly differentiated NSCLC when LCNEC is a possibility in the differential diagnosis. When compared with p63, the nontransactivating isoform of the p63 gene, delta Np63 (p40), was found to be a more reliable marker for squamous differentiation. Expression of p40 was lower compared with p63 in LCNEC and therefore more accurate. These results were confirmed in a study subtyping LCC with IHC; none of the confirmed LCNECs stained for p40.
Markers for Differentiating Large Cell Neuroendocrine Carcinoma From Adenocarcinoma
Thyroid transcription factor-1 (TTF-1), a marker commonly used in defining the appropriate histotype of lung cancer and that is also sensitive and specific for lung adenocarcinoma, is expressed in about 50% of all resected lung LCNEC. The expression ranges of TTF-1 mainly depend on the antibody used. The more sensitive clone, SPT24, is positive in 23% to 77% of LCNECs, and the 8G763 clone is positive in 23% to 48%. Therefore TTF-1 is not useful in differentiating LCNEC from poorly differentiated NSCLC (adenocarcinoma).
A new marker named Napsin-A, specific to adenocarcinomas, was negative in 100% of LCNECs, but further validation of this marker is required. In addition, collapsin response mediator protein (CRMP) is known to be involved in neurogenesis and was studied in a group of lung tumors. CRMP was expressed in 4 (100%) of 4 LCNECs and in 54 (100%) of 54 SCLCs. The expression of CRMP was negative in 0% of 22 adenocarcinomas and positive in 1 (8%) of 12 squamous cell carcinomas. The number of LCNECs studied was very small, but CRMP was shown to be a promising marker that also requires further validation.
Markers for Differentiating Bronchopulmonary Carcinoid From Small Cell Lung Cancer
Ki-67 staining may be of value because SCLC shows a proliferation index of more than 50%, whereas carcinoids have a proliferation index of less than 20%. K homology domain (KOC) protein and paired box gene 5 (PAX5) expressions have been evaluated in small samples. The KOC protein was strongly positive in 90% of SCLCs and negative in 20 of 21 cases of typical and atypical carcinoids. PAX5 expression was present in 29 (78%) of 37 high-grade neuroendocrine tumors and 0 of 51 typical and atypical carcinoids. Validation in larger samples is required before such markers are used routinely for differentiation.
Markers for Differentiating Large Cell Neuroendocrine Carcinoma From Small Cell Lung Cancer
As of the time of publication, distinguishing LCNEC from SCLC by IHC is not feasible. Several markers have been proposed, but lack practical usefulness. Both CK7 and 18 are commonly expressed in LCNEC and were reported in several studies to have a considerably higher expression intensity in LCNEC compared with SCLC. These results were similar in studies comparing the expression intensity of E-cadherin and β-catenin in LCNEC and SCLC, where both markers were shown to have considerably higher expression intensity in LCNEC. Villin1, a promising marker located in the brush border of epithelial cells, was found to be expressed in 62% of LCNECs and 4% of SCLCs. However, these results must be confirmed in larger studies. In a smaller study, IHC expression of neuronatin was examined in LCNEC and SCLC after an increased complementary DNA transcription of neuronatin in LCNEC was noted. Among the SCLC samples, 8% were positive for neuronatin with IHC staining compared with 43% among the LCNEC samples. A neurogenesis-regulating gene (NeuroD) was also found to be differentially expressed between LCNEC and SCLC. NeuroD was expressed in 53% of LCNECs and 13% of SCLCs. Therefore several markers may differentiate LCNEC from SCLC, but none of these is very specific or sensitive and further validation is required.
Markers for Differentiating Atypical and Typical Carcinoids From Large Cell Neuroendocrine Carcinoma
Separating atypical and typical carcinoids from LCNEC is possible with the Ki-67 index. The expression index in LCNEC is approximately 40% (range, 25% to 52%); for carcinoids, the expression index is reported to be below 20% (typical carcinoid, less than 2%; atypical carcinoid, less than 20%, typically ± 10%). Consequently, Ki-67 may be useful in differentiating LCNEC from atypical and typical carcinoids, although the diagnosis has to be confirmed with a mitoses count.
Immunohistochemical on Cytology
IHC neuroendocrine markers such as chromogranin, synaptophysin, and NCAM were found to be positive in 28% to 31%, 64% to 75%, and 45% of cytologic smears, respectively.
Molecular Biology
Approximately 5% of BP carcinoids may occur as a result of the multiple neuroendocrine neoplasia type 1 gene (MEN1); 95% are sporadic tumors of uncertain etiology. Various techniques have been used to evaluate the molecular biology of BP carcinoids, including in vitro cell line studies, chromosomal evaluation, comparative genomic hybridization (CGH), polymerase chain reaction, and, most recently, whole genome sequencing. Swarts et al. performed an extensive review of the molecular biology of neuroendocrine lung tumors, and Cakir and Grossman examined the molecular pathogenesis of BP carcinoids. The molecular basis of LCNEC is also still unknown. SCLC and LCNEC are known as highly undifferentiated pulmonary tumors that express similar characteristics. However, according to WHO classification, LCNEC is categorized as a subtype of LCC. The molecular biology of LCNEC, determined by different techniques, has been addressed in several studies and the findings were compared with SCLC and LCC.
Loss of Heterozygosity
BP carcinoids may have loss of heterozygosity (LOH) on a variety of different chromosomes. LOH at 11q has been seen in 27% to 55.5% of typical carcinoids and 0% to 73% of atypical carcinoids. LOH at 11q13, the locus of the MEN1 gene, was detected in 50% to 63.6% of atypical carcinoids and 22% to 73% of typical carcinoids. In several other small studies, LOH was found on several chromosomes in bronchial carcinoids, including 3p14.2 (fragile histidine triad gene); 5q;67 5q21 (adenomatous polyposis coil/mutated in colon cancer gene);68 9p;67,70 9p21 (p16);68 9q;67 13q;67 13q14.1–14.2 (retinoblastoma [Rb]);68 17p;67 17q13.1 (p53); and X (microsatellite markers).
In an evaluation of LOH with microsatellite markers at chromosomes 3p, 5q, 9p, 11q, and 13q, several similarities between SCLC and LCNEC were demonstrated. These chromosomal findings were in accordance with the results from another study that compared LOH in LCC, LCNEC, and SCLC. LOH was examined for chromosomes 3p, 5q, 9p, 10p, 10q, and 13q. Significant differences were noted between all three tumor types except for SCLC and LCNEC, emphasizing the close relation between these high-grade neuroendocrine tumors.
LOH has also been examined in combined SCLC and LCNEC tumors. Depending on the region being examined, combined tumors can express an SCLC or LCNEC phenotype. Investigators separately studied SCLC and LCNEC regions and found a high degree of similarity in the genetic profile. These findings suggest that there is a common origin of combined SCLC and LCNEC tumors.
Chromosomal Aberrations
CGH and other genetic analyses to assess chromosomal aberrations in BP carcinoids, high-grade pulmonary NECs, or both have been examined in several studies. Swarts et al. performed a meta-analysis of these studies, which included typical carcinoids, 38 atypical carcinoids, 33 LCNECs, 48 SCLCs, and 11 unclassified high-grade neuroendocrine tumors. Chromosomal aberrations more than 10 Mb were much more frequent in LCNEC (average 13.7 aberrations per tumor) and SCLC (18.8 aberrations per tumor), as compared with atypical carcinoids (6.1 aberrations per tumor) and typical carcinoids (2.8 aberrations per tumor). The investigators assumed that smoking habit may explain the differences. The most frequent aberrations for bronchial carcinoids are –11q, +19P, –13q, +19q, +17q, –11p, –6q, +16p, +20p, and –3p. These aberrations differ from those present in the high-grade neuroendocrine tumors, with the exception of 3p and 13q losses.
Chromosomal aberration in LCNEC and SCLC has also been studied. Through CGH, loss of chromosomes 3p, 4, 5p, 6q, 8p, 9p, and 21q, and gain in 5p, 8q, 12p, and 22 was found in three patients with LCNEC. Similarities between chromosomal aberrations in LCNEC and SCLC included loss of 3p, 4q, 5q, and 13q and gain of 5p. Noticeable chromosomal aberrations between SCLC and LCNEC were found at chromosomal arms 3q (gain), 10q (loss), and 17p (loss) in favor of SCLC, and gain of 6p in favor of LCNEC. Compared with atypical carcinoid, no similarities were detected. In a study by Peng et al., several similarities between LCNEC and SCLC were found when the genetic profiles were analyzed with high-density bacterial artificial chromosome array. Frequently gained loci were located at 1q, 2q, 3q, 5p, 7q, 8q, 12q, and 18q; lost loci were located at 1p, 3p, 4q, 5q, 10q, 13q, 16q, 17p, and 22q. Considerably different chromosomal aberrations compared between all stages of SCLC and LCNEC were located at 2q (gain), 3p (loss), 4q (loss), and 6p (loss). Although it may appear that SCLC and LCNEC share a common genetic profile, the majority of similarities may not be very specific, because numerous chromosomal aberrations are commonly encountered in other forms of lung cancer, including pulmonary NEC.
Microarray Comparison
Anbazhagan et al. performed hierarchical clustering of gene expression profiles of two carcinoids, two SCLCs, and two brain tumors. Although the carcinoids clustered together with the brain tumors, SCLC was more closely related to normal bronchial epithelial carcinoma. Swarts et al. used gene expression profiling to explore pathways involved in lung carcinoid progression and found that in carcinoids with poor prognosis, a significantly higher number of downregulated genes were located at chromosome 11q ( p = 0.00017). In addition, a number of upregulated genes were found to be involved in the mitotic spindle checkpoint, the chromosomal passenger complex, mitotic kinase CDC2 activity, and the BRCA /Fanconi anemia pathway. At the individual gene level, BIRC5 (survivin), BUB1, CD44, IL20RA, KLK12, and OTP were independent predictors of patient outcome. For BIRC5, the number of positive nuclei was also related to poor prognosis within the group of carcinoids. Aurora B kinase and BIRC5, major components of the chromosomal passenger complex, were particularly upregulated in high-grade carcinomas and may therefore be indicative of therapeutic targets for these tumors.
When examining the clinical behavior of high-grade neuroendocrine tumors, classification based on genetic profiling may be more appropriate than histologic classification. Jones et al. examined complementary DNA microarray data obtained from neuroendocrine lung tumors (8 LCNECs [2 combined with SCLC], 17 SCLCs [2 combined with LCNEC], and 13 LCCs). Surprisingly, neither SCLC nor LCNEC clustered as single entities in the way that LCCs did. In support of this molecular description of neuroendocrine lung tumors, Shibata et al. found three high-grade neuroendocrine subclasses when data from comparative genome hybridization were hierarchically clustered (8 SCLC and 15 LCNEC). The three subclass branches were subdivided into groups named BR1, BR2, and BR3. Patients in the BR2 group had a significantly different survival compared with the BR1 and BR3 groups ( p = 0.028). However, contrary to findings from Jones et al., almost all SCLCs clustered.
Mutation Analysis
In a recent study by Sachithanandan et al. of patients with MEN1, 5% had been diagnosed with BP carcinoids. MEN1 is an autosomal dominant disorder associated with mutations in the gene locus on 11q13. MEN1 gene activation is evident in 70% of patients with atypical carcinoid, 47% with typical carcinoid, 52% with LCNEC, and 41% with SCLC. A single mutation was found in a small study of MEN1 in LCNEC.
Capodanno et al. assessed 190 patients with bronchial neuroendocrine tumors (75 typical carcinoids, 23 atypical carcinoids, 17 LCNECs, and 75 SCLCs) and found that there was an increasing frequency of phosphatidylinositol 3-kinase (PI3K) mutations, with increased biologic aggressiveness of the bronchial neuroendocrine tumors, except in LCNECs. PIK3CA mutations were present in 13% of typical carcinoids, 39% of atypical carcinoids, 31% of SCLCs, and only 12% of LCNECs.
Mutations in LCNEC that are commonly encountered in lung cancer have been examined in several studies ( Table 55.2 ). Kirsten rat sarcoma viral oncogene homolog (KRAS) and epidermal growth factor receptor mutations are uncommon in LCNEC, although there have been published reports in cases of combined LCNEC and adenocarcinomas. Aberrant expression of anaplastic lymphoma kinase (ALK) by IHC was demonstrated in a single case of LCNEC. No ALK rearrangement or mutation was noted in further analyses. Two PIK3CA mutations have been found at c.3145 G>A and c.3140 A>G. In 29% of resected LCNECs, a mutation in the neurotropic tyrosine receptor kinase gene family was demonstrated.
| Type of LCNEC (Number of Samples With Mutation/Total Number of Samples) | ||||
|---|---|---|---|---|
| Mutation | Pure | With SCLC | With Squamous Cell Carcinoma | With Adenocarcinoma |
| ALK | 0/106 b | ND | ND | ND |
| BRAF | 0/24 | 2/9 | 0/3 | ND |
| EGFR | 1/62 | 0/10 | 0/3 | 0/8 |
| KRAS | 2/83 | 1/10 | ND | 1/9 |
| NRAS | 0/34 | 0/6 | ND | ND |
| PIK3CA | 2/43 | 1/10 | ND | ND |
| ROS1 | ND | ND | ND | ND |
| HRAS | 1/17 | 0/8 | 0/3 | ND |
| MEN1 | 1/13 | ND | ND | ND |
| TP53 | 32/61 | 3/10 | ND | 1/1 |
| KEAP1 | 2/19 | 0/9 | 0/1 | ND |
| NTRK | 6/21 | ND | ND | ND |
| NFE2L2 | 1/19 | 0/9 | ND | ND |
| STK11 | 8/28 | 1/10 | ND | ND |
| CDK4 | 0/28 | 0/10 | 0/3 | ND |
| DDR2 | 0/1 | 0/2 | 0/1 | ND |
| ERBB2 | 2/26 | 0/10 | 0/3 | ND |
| FGFR2 | 1/24 | 0/10 | 0/1 | ND |
| FGFR3 | 0/25 | 0/9 | 0/2 | ND |
| C-Kit | 0/83 | ND | ND | ND |
| C-met | 0/83 | ND | ND | ND |
| PDGFR alpha | 0/83 | ND | ND | ND |
| PFGFR beta | 0/83 | ND | ND | ND |
In a 2013 study, a large group of molecularly analyzed lung tumors was reported by the Clinical Lung Cancer Genome Project and Network Genomic Medicine. A total of 261 lung tumors (31 LCCs) were included for unsupervised hierarchical clustering of gene expressions. Although the analyzed carcinoid tumors harbored no noteworthy mutations, a number of mutations were identified in pure LCNEC and the combination of SCLC and LCNEC ( Table 55.2 ). Based on genetic modeling, the authors of the study were able to classify the majority of all LCCs as adenocarcinoma, squamous cell carcinoma, or SCLC. Thereby, the diagnosis of LCC as a separate entity was brought into question. Whole-exome (15 tumors) and transcriptome (10 tumors) sequencing of LCNEC showed overlapping mutations between LCNEC and SCLC (T53, RB1, and EP300). Several mutations typically found in adenocarcinomas or squamous cell carcinomas were detected in LCNEC, but these findings were not significant. Additional information about the whole-exome and transcriptome sequencing of LCNEC is expected to be published in the near future.
Pathways
The p53 gene, which helps to maintain genomic stability, is mutated in approximately 4% of typical carcinoids, 29% of atypical carcinoids, 80% of LCNECs, and 75% of SCLCs.
The p16/cyclin D1/Rb pathway is affected in 9% to 20% of typical carcinoids, 22% of atypical carcinoids, 62% of LCNECs, and 71% to 90% of SCLCs. The Rb gene is a tumor suppressor with a critical role in cell cycle control through the regulation of the G 1 growth arrest. p16 is a cyclin-dependent kinase inhibitor that inhibits binding of cyclin-dependent kinases to cyclin D1 and prevents phosphorylation (inhibition) of Rb. Therefore downregulation of p16 and Rb may lead to uncontrolled cell growth. Loss of Rb expression was demonstrated in 47% to 91% of LCNECs. Loss of p16 expression and overexpression of cyclin D1 were found more frequently in LCNEC than in SCLC.
The intrinsic apoptosis pathway, including Bcl2, Bcl2L1, and BAX genes, has been found in several studies to be inhibited in high-grade neuroendocrine tumors, moderately affected in atypical carcinoids, and almost intact in typical carcinoids.
The expression of AKT and mammalian target of rapamycin (mTOR) as part of the PI3K/AKT/mTOR pathway in a group of neuroendocrine lung tumors has also been evaluated in several studies; however, there were contradicting results for AKT expression (19% to 82%) and mTOR expression (50% to 77%) in these tumors. Righi et al. described a pattern of mTOR intensity expression that decreased from low- to high-grade neuroendocrine tumors. The authors also noted that mTOR expression correlated with SSRT-2/3 expression and hypothesized that mTOR may be a possible regulator of SSRT expression.
Clinical Characteristics
BP Carcinoids
As a result of endobronchial obstruction and tumor ulceration, approximately 58% of patients with BP carcinoids present with symptoms including cough (32%), hemoptysis (26%), and pneumonia (24%).
Contrary to midgut carcinoids, BP carcinoids produce less serotonin and thus patients with BP carcinoids have a lower rate of carcinoid syndrome. Carcinoid syndrome, which occurs in 1% to 3% of BP carcinoid cases, may be atypical, with episodes of flushing accompanied by disorientation, tremor, periorbital edema, lacrimation, salivation, hypotension, tachycardia, diarrhea, dyspnea, asthma, and edema. In addition, the risk of carcinoid crisis with BP carcinoids is so low that routine prophylaxis with octreotide prior to tumor manipulation is not typically recommended.
Approximately 1% to 2% of BP carcinoids are associated with ectopic adrenocorticotropic hormone production. Even more rare is the production of growth hormone-releasing hormone from BP carcinoids causing acromegaly, hypoglycemia, and hypercalcemia.
Large Cell Neuroendocrine Carcinoma
Because of the difficulty in diagnosing LCNEC on small biopsy specimens and cytology samples, most clinical data come from surgical series, which may bias the results (i.e., selection of younger patients with less comorbidity). Reported symptoms at initial presentation often include coughing, weight loss, hemoptysis, chest pain, and fever. Classic paraneoplastic syndromes, such as Cushing syndrome seen in SCLC or the carcinoid syndrome rarely associated with lung carcinoids, are seldom diagnosed in LCNEC.
The incidence of LCNEC is about 3% in reviewed case series of resected pulmonary malignancies. In these LCNEC series, the average age of initial presentation was 64 years (range, 30 years to 88 years) with the majority of patients being male (mean, 80%; range, 54% to 89%) and former heavy smokers (89% to 100%; Table 55.3 ). Data from two studies have challenged that the incidence of LCNEC is substantially higher in men. In 100 cases of confirmed resected LCNEC, the female-to-male ratio was 5:9 (46%:54%). In a study of clinical characteristics extracted from the Surveillance, Epidemiology, and End Results database, the findings were similar, with a female-to-male ratio of approximately 5:9 (45%:55%).
| Characteristics | Takei et al. | Rossi et al. | Veronesi et al. | Varlotto et al. | Fournel et al. | Tanaka et al. | Sarkaria et al. | Grand et al. | Grand et al. | Kinoshita et al. | Asamura et al. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Period | 1982–1999 | 1990–2004 | 1988–2004 | 2001–2007 | 2000–2010 | 2001–2009 | 1992–2008 | 1980–2009 | 1980–2009 | 1995–2010 | NA |
| Pathology review board, number of pathologists | 3 | 3 | 1 at each center | No review | 1 | 1 | 2 | NA | NA | 3 | Central, 6 |
| No. of centers | 1 | 2 | Multicenter | Multicenter | 1 | 1 | 1 | 2 | 2 | 1 | Multicenter |
| No. of pure LCNEC (%) | 82 (94) | 83 | 144 | 324 | 63 | 63 | 77 (77) | 52 | 56 (69) | 126 (89) | |
| No. of combined LCNEC (%) | 5 (6) | — | — | — | — | — | 23 (23) | — | 50 | 25 (31) | 15 (11) |
| Men (%) | 86 | 88 | 81 | 55 | 77 | 87 | 54 | 71 | 86 | 85 | 89 |
| Mean age (y) | 62 | NR | 63 | 67 | 64 | 67 | 64 | 60.5 | 61.4 | 70 | 66 |
| Smokers (%) | 98 | 96 | 94 | NR | 89 | 92 | 98 | 95 | 94 | 100 | 99 |
| Surgery (%) | |||||||||||
| Segment/wedge resection | 9 | NR | 10 | 22 | 6 | 13 | 9 | 13 | 6 | 0 | NR |
| Lobectomy | 70 | NR | 66 | 73 | 73 | 87 | 80 | 63 | 48 | 95 | NR |
| Bilobectomy | 7 | NR | 5 | NR | 1 | 0 | 0 | 4 | 6 | 0 | NR |
| Pneumonectomy | 14 | NR | 17 | 6 | 19 | 0 | 11 | 19 | 40 | 5 | NR |
| Systematic node dissection (%) | Yes (69) | Yes | Yes (94) | NR | Yes | NR | NR | Yes | Yes | Yes | NR |
| R0 resection (%) | NR | NR | 94 | NR | 100 | 100 | 90 | 96 | 86 | NR | NR |
| Lymph Node Status (%) | |||||||||||
| N0 | 49 | 75 | NR | 69 | 46 | NR | 65 | 52 | 58 | NR | 54 |
| N1 | 20 a | 25 a | NR | 17 | 24 | NR | 15 | 19 | 22 | NR | 18 |
| >N2 | 20 a | 25 a | NR | 12 | 30 | NR | 23 | 29 | 20 | NR | 26 |
| Stage (%) b | |||||||||||
| I | 47 (4) | 66 (6) | 51 (NR) | 57 (NR) | 35 (7) | 45 (6) | 44 (7) | 38 (7) | 40 (7) | 59 (7) | 45 (5) |
| II | 15 (4) | 20 (6) | 20 (NR) | 16 (NR) | 25 (7) | 26 (6) | 27 (7) | 25 (7) | 28 (7) | 21 (7) | 16 (5) |
| III | 34 (4) | 14 (6) | 28 (NR) | 18 (NR) | 40 (7) | 19 (6) | 25 (7) | 33 (7) | 26 (7) | 19 (7) | 32 (5) |
| IV | 3 (4) | 0 (6) | 1 (NR) | 0 (NR) | 0 (7) | 0 (6) | 4 (7) | 2 (7) | 4 (7) | 1 (7) | 7 (5) |
| Adjuvant chemotherapy (%) | 14 | 34 | 17 | NR | Yes (% NR) | 32 | 25 | NR | NR | Yes (% NR) | NR |
| 5-Year survival (%) | 57 | 27.6 | 43 | 41 c | 49.2 | 44.9 | NR | 39 | 38 | 53.3 | 40.3 |
| 5-Year Survival by Stage (%) | |||||||||||
| I | 67 | 33 | 52 | 60 c | NR | NR | 53 | NR | NR | NR | 58 |
| II | 75 | 23 | 59 | NR | NR | NR | 61 | NR | NR | NR | 32 |
| III | 45 | 8 | 20 | NR | NR | NR | 24 d | NR | NR | NR | NR |
| IV | 0 | — | — | — | NR | NR | 24 d | NR | NR | NR | NR |
| Median follow-up (mo) | NR | 17 | 27 | 15 | NR | 32.3 | 34 | 73 e | 73 e | 60 | 60 |
| Recurrence (%) | 39 | 62 | 40 | NR | NR | NR | 38 | NR | NR | NR | 48 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree