Humans lose the regenerative potential for wound repair early in embryonic life and following that wounds heal by scarring. Although scarring is a protective mechanism that maintains tissue continuity, it has a major adverse impact on health and society, as we witness in myocardial infarction, stroke and post-burn scar contractures, etc. Recently, regenerative medicine and tissue engineering have emerged with the hope of achieving healing by regeneration rather than repair and scarring.
Tissue engineering applies the principles of biology and engineering to the development of functional substitutes for damaged tissue.1 There are three main pillars of tissue engineering: a scaffold, cells, and biological inducers that facilitate cellular migration and growth. Scaffolds are templates that are made of collagen or synthetic polymers and act as a framework for cellular growth. Most of the available skin substitutes are formed only of an acellular template that acts as a scaffold.
As patients are increasingly surviving larger burns due to the introduction of early total burn excision, the need for a wound cover material more durable than allografts or xenografts became clear. This resulted in the collaboration of Yannas, a scientist at the Massachusetts Institute of Technology (MIT), and John Burke, a surgeon at the Massachusetts General Hospital and the Shriner’s Burns Centre in Boston, in the 1970s, to produce the first regenerative scaffold to be commercially produced: Integra®. A multicenter clinical trial was conducted and published in 1988, followed in 1990 by a histological study of the phases of wound healing and repair in the same group of patients.2,3 Integra is now widely used in acute burns and reconstructive surgery4 since the U.S. Food and Drug Administration (FDA) granted the manufacturer a license in 1996. In 2003, the license was extended to include the use of Integra for full-thickness wound cover. This widened the indications for the use of Integra to include post-burn reconstruction,4,5 trauma,6,7 skin oncology,8 chronic wounds,9 and a host of other applications.10–13 Other skin substitutes have since been introduced. In the late 1970s, Bell et al14 started developing a new type of skin substitute matrix that was bilayered to mimic the epidermal and dermal layers of skin and which housed living fibroblasts and epidermal cells. This led to the development of the bilayer skin substitute Apligraf (formerly known as Graftskin) by Organogenesis, Inc. that received FDA approval in 2000 for use in the treatment of venous and diabetic ulcers. A similar skin substitute, Orcel (Ortec International, Inc.) was granted FDA approval in 2001 for use in the treatment of fresh split-thickness autograft donor sites in burn patients. In 2000, a non-cross-linked collagen elastin matrix was produced by collaborative work in Europe led by Dr Otto Suwelack. This was later marketed as Matriderm and showed promising results in the treatment of burn wounds.15 Besides these examples, there are now many alternatives available, including AlloDerm (LifeCell Inc), Pelnac (Gunze Medical Division), and OASIS™ Wound Matrix (Cook Biotech) and many others.
The majority of skin regenerative scaffolds are currently collagen-based; however, there has been an emergence of new materials: natural16 and synthetic polymers. The techniques of producing scaffolds from these new materials have become very sophisticated, especially with the use of nanostructured materials. As the cost of current skin substitutes is prohibiting their wide clinical use, future generations of cheaper skin substitutes that also utilize progenitor or stem cells will benefit our patients.
Integra is currently the most commonly used skin substitute. It is a dermal template that consists of two layers: a porous bovine collagen blended with chondroitin-6-sulfate glycosaminoglycan and a synthetic silicone polymer forming the epidermal substitute.1 The bovine collagen acellular matrix becomes populated with fibroblasts and endothelial and other cells from the wound bed. The integration of the skin substitutes into the wound bed is similar and can be divided into four stages similar to the stages of skin graft take and wound healing.
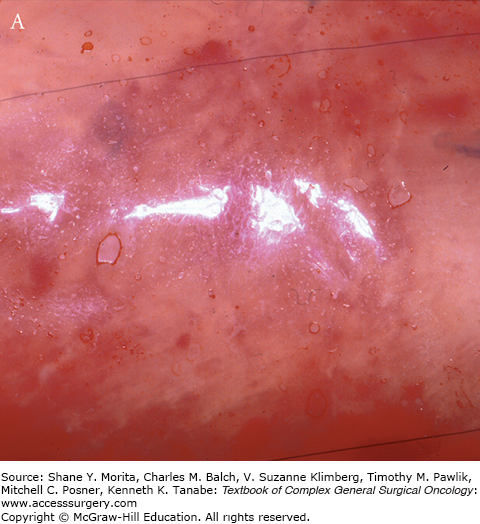
This occurs within minutes of applying Integra onto the wound. Following the application of the matrix, the interstices fill with wound fluids that contain red blood cells. Fibrin from the wound assists in adherence of the matrix to the wound bed. The appearance of the matrix is a reflection of the above changes (see Fig. 160-1).
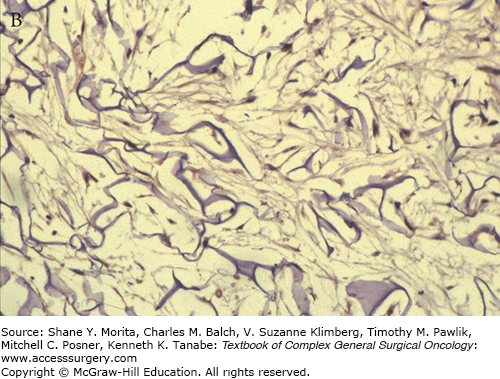
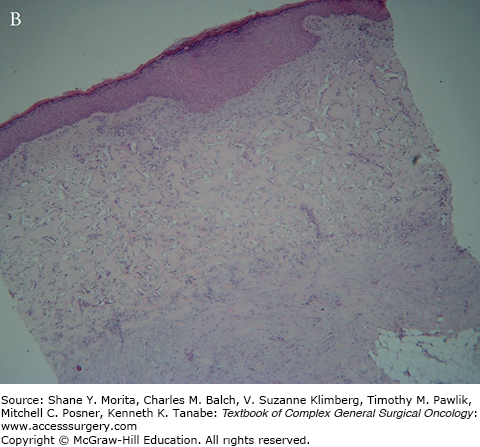
This stage begins with the appearance of fibroblasts, characterized by spindle-shaped cells with abundant cytoplasm and oval nuclei with plump nucleoli by day 3 at the base of the matrix. By the third week the cells settle along the interstices of the whole thickness of the matrix and start producing host collagen (see Fig. 160-2).
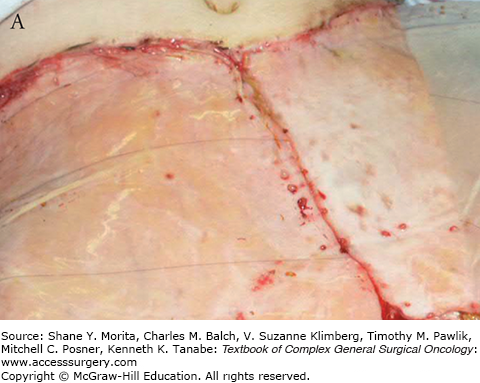
Starting toward the end of the second week, endothelial migration is observed marking the beginning of neovascularization. Lumen formation is seen in the third week. At week 4, fully canalized endothelial channels are seen throughout the width of the matrix. It is at this stage that the matrix bed is optimum for “taking” a graft (see Fig. 160-3).
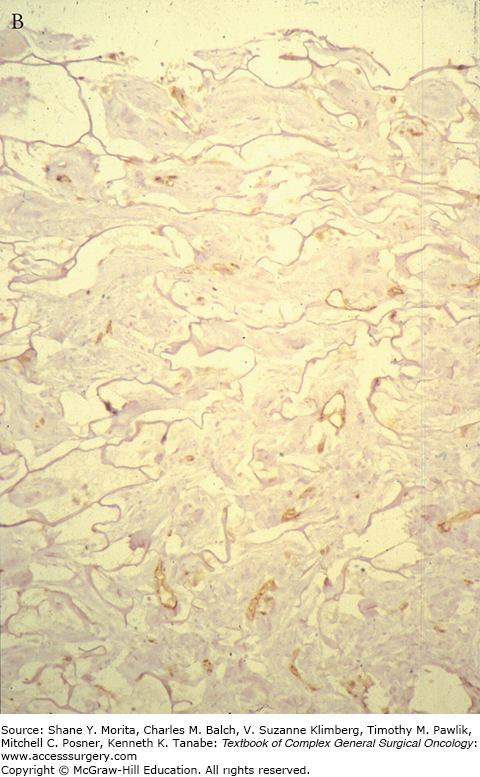
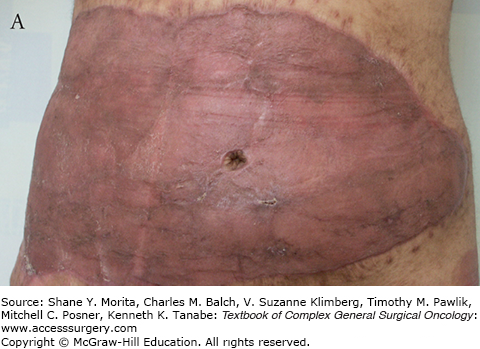
As the migrating host, fibroblasts settle, proliferate, and start producing extracellular matrix, mainly collagen or “neo-collagen.” The interstices become gradually filled forming neodermis. As the collagen deposition continues, the neodermis becomes thicker and clinically red in color. The thickness and the redness can take months to resolve, longer than skin grafts. The neodermis collagen is well organized and is indistinguishable from normal dermal collagen; however, there is a distinctive absence of adnexal structures even on the long term (see Fig. 160-4).
Nerve endings and some elastic fibers were demonstrated within the matrix more than 2 years after the application of Integra. Few remnants of the bovine collagen matrix were also present within the neodermis 18 months postengrafting. However no adnexal structures were shown.
Skin substitutes have to be carefully considered together with other options of reconstructions.
Reconstruction with dermal skin substitutes offers several advantages over other reconstruction methods.
In the elderly, complicated reconstructive procedure would have the added risk of prolonged surgery and anesthesia especially for the medically unfit patient, whereas reconstruction with skin substitutes can be safely performed under local anesthesia as day cases in appropriate cases.
Skin substitutes, particularly bilaminar, offer a reliable cover of exposed structures where skin graft would not be suitable. Exposed bone and tendons are examples. This is useful after wide/deep excision of tumors or following trauma.
A suboptimal wound bed that would not support skin graft or even a flap can be adequately covered by bilaminar skin substitutes such as Integra. Previously irradiated17–19 or contaminated wounds13 were shown to be successfully covered by Integra. When wound bed vascularization is compromised, the Integra matrix is vascularized slowly. The silicone layer protects and preserves the matrix until the neodermis is vascularized well enough to support the engraftment at the second stage. The second stage is often delayed for 8 to 10 weeks in these cases.19,20 When ready, the silastic (silicone) layer is removed and the underlying neodermis is grafted.
Costs of skin substitutes are high compared to the alternative treatments.
For skin substitutes requiring two stages, for example, Integra, patients will usually require weekly inspections of the Integra for at least 4 weeks. There is a steep learning curve for the whole multidisciplinary team to ensure the smooth postoperative care pathway.
As skin substitutes have no antimicrobial properties, if the wound bed is heavily contaminated the matrix presents an ideal condition for bacterial growth.
As a majority of skin substitutes consist of bovine- or porcine-derived components, patients with known allergies or related ethical/religious concerns should be appropriately informed. Skin substitutes harvested from human donors (e.g., AlloDerm) also carry a theoretical risk of disease transmission, which has been proven to be negligible based on the lack of documented occurrences in the world’s literature.
Short shelf life and specialist storage: Some skin substitutes, if contain viable cells, have very short shelf lives (e.g., Apligraf has a 15-day shelf life) or require special storage conditions (e.g., Orcel requires cryopreservation).
Among its wide range of protective, perceptive, and regulatory functions, the human skin’s role of providing a protective barrier is the most crucial for survival.1 Bilaminar skin substitutes have spread from their initial use in burns and are now widely utilized for a variety of skin defects, which are described below.
Skin substitutes are particularly useful in the elderly or as an alternative to free or local flaps when other reconstruction methods have been exhausted or are unsuitable. Dermal substitutes are most commonly used for reconstruction after the excision of skin malignancies such as basal cell carcinomas, squamous cell carcinomas, and melanomas.8,19–22
Integra, and to a lesser extent other skin substitutes, has established an important role in wound coverage after acute burn and post-burn reconstruction of scarring and contractures.23,24
Traumatic wounds are often contaminated and may contain devitalized tissues; however, these wounds can be perfectly covered with Integra after the wound has been adequately debrided and possibly treated with negative pressure therapy (NPT) to prepare the wound bed for Integra. It is thought that because skin substitutes such as Integra are acellular, the matrix is not reliant on immediate vascular ingrowth. Thus, exposed tendons, bones lacking periosteum, and open small joints can and have been shown to be efficiently covered by skin substitutes, especially with two-stage procedures in a number of case series13,25,26 and a systematic review.27
Most dermal substitutes have become increasingly used in the treatment of various nonhealing ulcers.11,12,28 Dermagraft and Apligraf are some examples of skin substitutes, which have been approved by the FDA for use in reconstructing venous and diabetic ulcers.
Congenital skin conditions present a significant challenge to the reconstructing surgeon due to the large surface area they affect that require extensive donor site. Giant nevi, left unexcised, present a risk of malignancy and previously have been amenable to serial excisions and multiple skin grafts with suboptimal outcomes. Integra offers quality and durable reconstruction that has been shown to be superior to partial-thickness skin grafting.29
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree