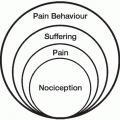
Desire for hastened death among terminally ill cancer patients is not uncommon.
1,
2 Depression and hopelessness are predictors of desire for hastened death in this population and provide independent and unique contributions. Interventions addressing depression, hopelessness, and social support are important aspects of adequate palliative care, particularly as it relates to desire for hastened death. Miovic and Block
3 reported that approximately 50% of patients with advanced cancer meet criteria for a psychiatric disorder, the most common being adjustment disorders (11% to 35%) and major depression (5% to 26%).
Treatment decisions for depression in the cancer patient presume that a thorough medical and psychiatric assessment has led to an accurate diagnosis that will allow specific and effective intervention. Tumor involvement of the CNS, hyperparathyroidism, and adrenal insufficiency may contribute to mood disorders. Patients with severe depression and/or suicidal ideation or intent require psychiatric consultation. Before planning an intervention, the physician should consider a history of previous depressive episodes and substance (including alcohol) abuse, family history of depression and suicide, concurrent life stresses, losses secondary to cancer (eg, financial, social, and occupation) in addition to loss of good health, and the availability of social support. Once it becomes clear that the patient is depressed, one must consider coexisting organic factors before starting treatment.
Standard therapeutic approaches such as the use of corticosteroids,
4 chemotherapeutic agents (tamoxifen,
5 asparaginase,
6 interferon,
7 and interleukin),
8 whole brain radiation,
9 and amphotericin
10 can cause or exacerbate depressive symptoms in cancer patients (
Table 24.1). Likewise the presence of brain metastases or tumors,
11 metabolic and endocrine complications,
12 and paraneoplastic syndromes
13 often contribute to the presence of depressive symptoms.
Depressed patients with cancer should be treated with a combination of psychological treatments (cognitive behavioral and supportive psychotherapy) and psychopharmacological interventions (antidepressants and psychostimulants). For patients with cancer pain, interventions that help diminish mood disturbance also help reduce pain.
14 Reducing severe pain may help relieve depression. In cancer patients with depressive disorders or with significant depressive symptoms, studies thus far provided only modest evidence for the benefit of pharmacological and psychosocial interventions.
2
PSYCHOTHERAPY
The goals of psychotherapy are to reduce emotional distress and to improve morale, coping ability, self-esteem, sense of control, and resolution of problems. It consists of four basic components—social support, emotional expression, cognitive restructuring, and coping skills training. Different formats for psychosocial support and therapy exist, including psychoeducation, cognitive-behavioral therapy, supportive therapy, and individual therapy, each of which may be more useful depending on the stage of illness and the individual’s attributes and circumstances. Although psychotherapy can be an effective treatment for depression in cancer patients, pharmacotherapy is frequently needed, because terminally ill patients often cannot participate fully in psychotherapy.
ANTIDEPRESSANT MEDICATIONS
Antidepressant medications should be considered for the treatment of moderate to severe major depression in cancer patients. Current evidence does not support the relative superiority of one pharmacologic treatment over another, nor the superiority of pharmacologic treatment over psychosocial interventions.
2 The choice of an antidepressant should be based on individual medication and patient factors: the side effect profiles of the medication, tolerability of treatment (including the potential for interaction with other current medications), response to previous treatment, and patient preference. Antidepressants commonly used in cancer patients are listed in
Table 24.2.
The side effect profile with the newer antidepressants, including the selective serotonin reuptake inhibitors (SSRIs) and the mixed-action antidepressants appear to show fewer side effects than the tricyclic antidepressants (TCAs) and monoamine oxidase inhibitors (MAOIs). The SSRIs and mixed-action antidepressants should be considered first-line treatments for depression in cancer patients. Commonly used SSRIs include fluoxetine, sertaline, paroxetine, fluvoxamine, citalopram, and escitalopram. The most common side effects associated with the SSRIs
are nausea, headache, sleep disturbance, sexual dysfunction, appetite suppression, and anxiety with the first few days of use. There are important differences in these agents including half-life, drug interactions, and individual side effect profiles. Fluoxetine, for example, has the longest half-life resulting in little if any risk of SSRI discontinuation syndrome with abrupt withdrawal. Because of the relatively short half-life (< 24 hours) of the other SSRIs, patients are at risk for developing significant psychiatric, neurologic, gastrointestinal, or flulike symptoms after abrupt withdrawal. All SSRIs are equally efficacious, with depressive symptoms improving 2 to 4 weeks after a therapeutic dose. Paroxetine causes the most anticholinergic side effects of the SSRIs and causes the most weight gain. Fluvoxamine and paroxetine are the more sedating, whereas fluoxetine is the most stimulating. Sertraline, citalopram, and escitalopram have fewer drug interactions, whereas fluvoxamine has the most, and this feature often limits its use.
TCAs act on multiple receptors (including muscarinic, cholinergic, histaminic, and adrenergic receptors) and side effects include dry mouth, constipation, confusion, urinary retention, sedation, weight gain, and orthostatic hypotension. These agents are potentially helpful for cancer patients with insomnia, pain, and depression and can be effective in relatively low doses. Dosing may be initiated at 10 to 25 mg at bedtime and the dose increased every 2 to 3 days until benefit is achieved. The effects on appetite and sleep are frequently very rapid; the effects on mood may be delayed or not clinically apparent. The choice of TCA depends on the nature of the depressive symptoms, coexistent medical problems, and side effects of the particular drug. The depressed patient who has insomnia may benefit from the use of a TCA that has sedating properties, such as amitriptyline or doxepin. Patients with psychomotor slowing will benefit from agents with the least sedating properties, such as desipramine. Patients with stomatitis secondary to chemotherapy or radiation therapy, or who have slow intestinal motility should receive agents with the least anticholinergic effects such as desipramine or nortriptyline. Imipramine, doxepin, amitriptyline, desipramine, and nortriptyline have been used in the management of neuropathic pain in cancer patients.
15 Dosing is similar to the treatment of depression, and analgesic efficacy, if it occurs, is usually observed at a dose of 50 to 150 mg daily; higher doses are sometimes necessary.
The novel and mixed-action antidepressants (venlafaxine, duloxetine, bupropion, trazodone, and mirtazapine) differ from the SSRIs in their mechanism of action, resulting in their different side effect profiles. Venlafaxine and duloxetine are serotonin/norepinephrine uptake inhibitors, with venlafaxine inhibiting serotonin reuptake at lower doses, thereby sharing some of the side effects of the SSRIs while inhibiting norepinephrine reuptake at higher doses. At higher doses (>225 mg/day) venlafaxine may contribute to hypertension. Both duloxetine and venlafaxine have been shown to improve neuropathic pain and peripheral neuropathy in cancer patients. Bupropion is primarily a noradrenergic agent that increases dopamine reuptake at higher doses. Its stimulating effects may be beneficial to the depressed cancer patient with fatigue. Bupropion has fewer gastrointestinal side effects than the SSRIs and tends to cause constipation more frequently than nausea, vomiting, or diarrhea. It increases the rise for seizures at higher doses and should be avoided or used
with caution in patients with seizure disorders or organic brain pathology. Venlafaxine, trazodone, mirtazapine, and SSRIs are useful in managing hot flashes.
16,
17 Mirtazapine is a noradrenergic and specific serotonergic antidepressant.
18 It has low affinity for muscarinic, cholinergic, and dopaminergic receptors, but a high affinity for H1 histaminic receptors. It antagonizes serotonin (5-HT) 5-HT
2 and 5-HT
3 receptors, resulting in increased serotonin release through 5-HT
1. The drug’s activity at these receptors may result in a variety of features, including sedation, anxiolysis, appetite stimulation, and antiemesis. These clearly have potential advantages in the oncology population.
PSYCHOSTIMULANTS
Psychostimulants such as dextroamphetamine (Dexedrine), methylphenidate (Ritalin), dexmethylphenidate (Focalin), pemoline (Cylert), and modafinil (Provigil) help diminish sedation related to opioids and are useful antidepressants in the medically ill. Concerns about hepatotoxicity and liver failure
19 led to the discontinuation of pemoline in May 2005 in the United States. Advantages in the use of psychostimulants in the cancer patient include a decrease in fatigue, improved concentration and attention, and appetite stimulation. Potential issues with the use of psychostimulants include nightmares, agitation, insomnia, and lower seizure threshold. The primary role of psychostimulants in advanced cancer is the treatment of symptoms such as cancer-related fatigue, opioid-induced sedation, depression, and cognitive dysfunction.
Methylphenidate is a piperidine-derived CNS stimulant structurally related to amphetamines. Dexmethylphenidate (Focalin), the d-enantiomer of methylphenidate, may have a role in the treatment of cancer-related fatigue. Dexmethylphenidate has approximately 6-hour duration of effect (the XR version spans 12 hours, covering a full day). Methylphenidate has binding affinity for both the dopamine transporter and norepinephrine transporter, with the D-isomer displaying a prominent affinity for the latter. After standard oral doses, peak plasma levels occur in 1 to 3 hours with a plasma half-life of 1.5 to 2.5 hours. Side effects include difficulty sleeping, stomachaches, headaches, lack of hunger (leading to weight loss), and dry mouth. Methylphenidate is available in 5-, 10-, and 20-mg tablets, as well as a 20-mg sustained-release tablet in which the drug is embedded in a wax matrix. Sustained-release methylphenidate has a longer T
max (3 to 4 hours) and T
1/2 (4 hours). Methylphenidate is also available as a transdermal patch. Approved uses for methylphenidate include attention deficit hyperactivity disorder and narcolepsy. Hypoactive delirium unexplained by an underlying cause (metabolic or drug-induced) and depression in patients with advanced cancer can be improved by the administration of methylphenidate.
20,
21 In 30 patients with malignant gliomas treated with methylphenidate, improvements in cognitive functioning and performance status were demonstrated despite ongoing neurologic injury due to disease progression or radiation therapy in half of these patients.
22 In a randomized, double-blind, placebo-controlled crossover trial of 32 patients with advanced cancer receiving chronic opioid therapy, statistically significant reductions in pain intensity and sedation were seen with the use of methylphenidate.
23 Further benefits on opioid sedation were also observed but tolerance to this effect was seen over a period of one month.
24Dextroamphetamine is a dextrorotatory stereoisomer of amphetamine and is also called dexamphetamine. The mechanism of action is unknown. In animals, it blocks dopamine and norepinephrine reuptake from central presynaptic neurons, inhibits MAOI activity, and facilitates catecholamine release. Dextroamphetamine also has peripheral α and β sympathomimetic actions, including elevation of both diastolic and systolic blood pressure. Dextroamphetamine (0.215 mg/kg) antagonized respiratory depression caused by low-dose morphine (0.15 mg/kg) over a 5-hour period as measured by CO
2 response curves and isohypercapnic minute ventilationin healthy volunteers, but did not reverse respiratory depression at higher doses of morphine (0.3 mg/kg).
25 Mitler et al.
26 suggested that both dextroamphetamine and methylphenidate were effective in treating somnolence in narcolepsy patients. Methamphetamine is subject to abuse and concerns have been expressed on the misuse of methamphetamine particularly in youths and young adults.
27 Treatment with dextroamphetamine or methylphenidate usually begins with a dose of 2.5 mg on awakening and at noon. The dosage is slowly increased over several days until a desired effect is achieved or side effects (overstimulation, anxiety, insomnia, paranoia, and confusion) become apparent. Occasional doses of 60 mg per day are required, but doses of not more than 30 mg per day are more typical.