Delivery Devices for Inhaled Medications
Umbreen S. Lodi
Theodore M. Lee
History of Inhalation Therapy
Inhalation therapy for bronchial disorders has been used since ancient times. Centuries ago, the stramonium (a botanically derived antimuscarinic agent) cigarette was described as a treatment for acute asthma (1,2). Antecedents of contemporary inhalation therapy are grounded in the early part of the 20th century with the invention of the hand-held glass bulb nebulizer (3), followed by introduction of compressor-driven nebulizers (4). Inhalation therapy subsequently was revolutionized by the introduction of pressurized metered-dose inhalers (MDIs) containing isoproterenol or epinephrine into clinical practice in the 1950s (5). In Europe, albuterol MDIs were first marketed in 1969 and beclomethasone dipropionate MDIs in 1972. The first dry powder inhaler (DPI), containing cromolyn sodium, was launched in 1967 (6,7).
Inhalation devices in use today include conventional pressurized MDIs (used with or without spacers or breath-actuation accessories), DPIs, and nebulizers.
Aerosol Particle Characteristics
A synopsis of the significance of particle behavior in inhalation therapy is a necessary preface to a clinically oriented discussion of aerosol devices. Deposition of aerosolized particles occurs as a result of inertial impaction, sedimentation, and diffusion (Table 37.1). The aerodynamic size of a particle describes its impaction and sedimentation characteristics and is the primary determinant of its capture in the airways. (8,9). Generally, the desirable aerodynamic size for pharmaceutical aerosols is 2 μm to 5 μm. Particles larger than 5 μm penetrate into the bronchi poorly, but potentially are absorbed systemically if swallowed. Particles under 2 μm are not deposited into the airways and are exhaled or are deposited in the alveoli (10,11) (Table 37.2) with minimal if any clinical benefit in the usual applications, but with occurrence of undesirable systemic absorption. The term fine particle mass is used for the percentage of the emitted dose that is in the respirable range, less than or equal to 5 μm (12). With most devices for aerosol therapy, fine particle mass is 10% to 25%. Deposition into peripheral airways relative to central airways is maximal at 2 μm to 3 μm (13).
Table 37.1 Mechanisms of Aerosol Particle Deposition | ||||||||
|---|---|---|---|---|---|---|---|---|
|
Table 37.2 Deposition of Aerosols | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Metered-Dose Inhalers
Propellants
Until recently, the propellants used in pressurized MDIs were chlorofluorocarbons (CFCs) known as Freon compounds. Manufacture of Freon compounds has been discontinued because of their role in depletion of the stratospheric ozone layer (14).
Chlorine free aerosol hydrofluoroalkane (HFA) propellants (15,16) which do not contribute to stratospheric ozone depletion have been developed. Because the surfactants used to stabilize suspensions in CFC MDIs are not compatible with HFA propellants, ensuring suspension stability was a significant challenge in the development of non-CFC MDIs (17). HFAs generally appear to be safe and effective alternatives to CFC propellants (18,19), although they are not necessarily absolutely interchangeable. For example, beclomethasone dipropionate (BDP) is in solution in HFA propellant, whereas CFC formulations of BDP were suspensions (20). BDP is at least twice as potent with HFA propellants, and the BDP HFA aerosol properties are more favorable for airway deposition (21). However, no significant differences in bronchodilating effects have been reported comparing CFC and HFA albuterol inhalers (14,22,23).
Some HFA MDIs contain excipients such as ethanol. Breath alcohol levels up to 35 μg per 100 mL have been detected for up to 5 minutes after 2 puffs of albuterol HFA MDIs containing ethanol (24). Because the infrared spectrums of HFAs overlap with commonly used
anesthetic gasses, HFA propellants may cause erroneous readings in anesthetic gas-monitoring systems (25).
anesthetic gasses, HFA propellants may cause erroneous readings in anesthetic gas-monitoring systems (25).
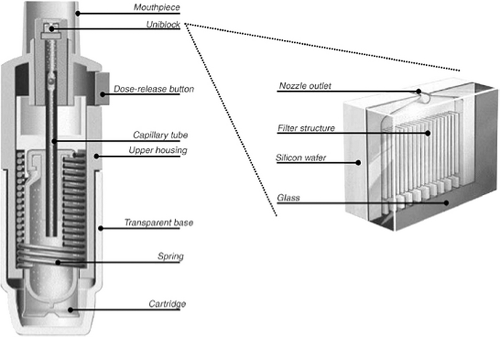
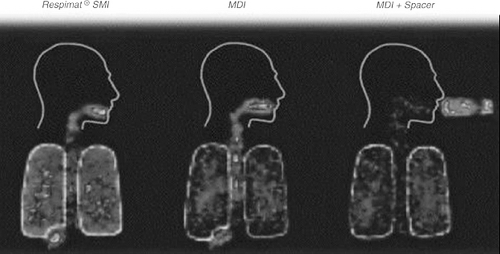
The Respimat Soft Mist inhaler (Boehringer Ingelheim Pharmaceuticals, Inc.—not available in the United States at this writing) is an MDI device which does not utilize pressurized propellants. It uses a spring-driven mechanism significantly differing from conventional pressurized MDIs in design ( Fig. 37.1) and output ( Fig. 37.2) (26).
Technique of Use of Metered-Dose Inhalers
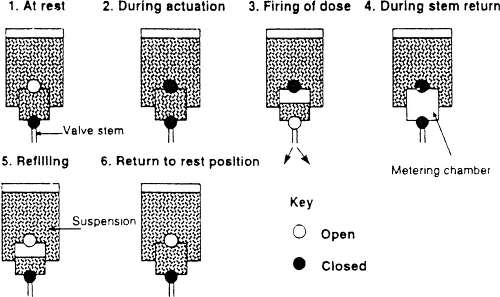
The pressurized MDI is comprised of several components including active drug, propellants/excipients, metering valve, actuator, and container ( Fig. 37.3). Figure 37.4 shows a schematic diagram of the operation of an MDI. Prior to actuation, the propellant-drug formulation for the subsequent single dose is contained within the small metering chamber inside the MDI canister. During the actuation, the metering chamber briefly communicates with the atmosphere but is sealed off from the remainder of the formulation within the canister; at this time, the dose within the metering chamber exits the inhaler through the valve stem. Immediately after the dose is released, however, the valve blocks the connection of the metering chamber to the atmosphere but permits the chamber to communicate with the interior of the canister, allowing refilling of the metering chamber.
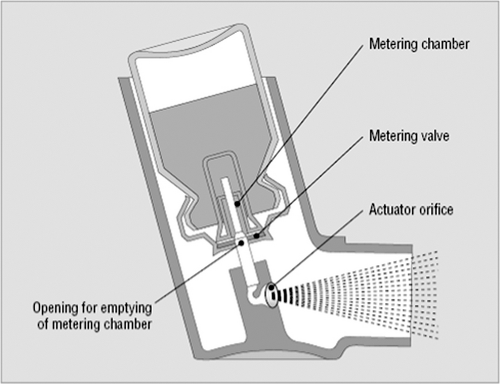 Figure 37.3 Schematic illustration of a typical pressurized metered-dose inhaler. (From Rees J. ABC of asthma—methods of delivering drugs. BMJ 2005;331:504–506; with permission.) |
In most cases the active drug is not soluble in the propellant; therefore, micronized drug particles within the canister are in suspension rather than in solution. Shaking the canister prior to each actuation is essential to ensure that drug particles are re-suspended; otherwise, the aliquot of propellant that enters the metering chamber may not be a homogenous suspension and therefore may not contain the expected amount of drug. Inattention to shaking a budesonide MDI prior to actuation has been documented to result in a significant reduction in drug delivery to the airways (27). If the pause between shaking and actuation is excessive, the drug-propellant suspension will become inhomogeneous before it is drawn into the metering chamber. Several other details are important for proper use of an MDI. The canister must be held still and in the vertical position until the valve has completely returned to its resting position. Failure to do so may cause inconsistent dosing. The likelihood of such problems increases toward the end of canister life (28); this issue is less significant with the re-engineered HFA inhalers which provide improved dosing consistency. When a period of time elapses between actuations of an MDI, the suspension that entered the metering chamber at the time of last actuation may lose homogeneity, with some drug sticking to the walls of the metering chamber. When this occurs, the amount of drug delivered with the next dose actuation will be reduced (29,30), a phenomenon termed loss of prime. The patient may compensate for this by discarding the first 2 puffs prior to each use of MDI, a procedure termed priming the inhaler (30). Loss
of prime was a significant issue with CFC MDIs; it is less significant with HFA MDIs (31). Breath holding increases drug deposition in the airways. Greater bronchodilation is found with a 10-second pause compared with a 4-second pause, but a 20-second pause appears to produce no further benefit (32).
of prime was a significant issue with CFC MDIs; it is less significant with HFA MDIs (31). Breath holding increases drug deposition in the airways. Greater bronchodilation is found with a 10-second pause compared with a 4-second pause, but a 20-second pause appears to produce no further benefit (32).
When an MDI is used without a holding chamber, the issue of whether the lips should be closed around the inhaler mouthpiece or instead held several centimeters from the open mouth (33,34) is open to debate. Comparisons between the two techniques have not shown consistent superiority of either technique over the other (32,35–37). Another issue relevant to optimal inhaler technique regards the lung volume at which inhalation begins. Although it has been proposed that inhalation from functional residual capacity yields improved results as compared with inhalation from residual volume (37), the difference is probably minor (39).
Limitations of Metered-Dose Inhalers as a Delivery System for Inhaled Medications
Many studies have documented the prevalence of errors in patients’ use of MDIs (40,41) (Table 37.3). Moreover, health care professionals often are not familiar with the details of appropriate use of the devices. Despite training, around 15% of individuals are not able to use inhalers properly without assistive devices. Of patients with initially inadequate technique who master proper technique with training, around 50% subsequently again develop significant deficiencies in technique over time (40). In patients with initially proper inhaler technique, 20% demonstrated incorrect usage at a later date. In addition to suboptimal response to treatment due to incorrect inhaler technique, there is significant direct economic loss as a result of wasted aerosol medication (41,42).
Table 37.3 Errors in Patient Use of Metered-Dose Inhalers | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||
Spacer Devices: Adjuncts to Metered-Dose Inhalers
Spacer devices are inhalation aids designed to overcome coordination difficulties, enhance aerosol deposition in the lower airways, and minimize oropharyngeal deposition (Fig. 37.5). There are three categories of spacers: (a) simple tubes which function as extensions of the actuator mouthpiece; (b) holding chambers with a one-way valve (43) to prevent the patient from blowing the dose away by exhaling into the chamber; and (c) reverse-flow reservoirs (44,45




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree