Definition, Diagnosis, and Classification of Diabetes Mellitus and Glucose Homeostasis
Peter H. Bennett
William C. Knowler
Diabetes mellitus is a heterogeneous group of metabolic disorders characterized by chronic hyperglycemia. Some forms of diabetes mellitus are characterized in terms of their specific etiology or pathogenesis, but the underlying etiology of the most common forms remains unclear. Regardless of the etiology, diabetes progresses through several clinical stages during its natural history. Persons developing the disease can be categorized according to clinical stages and other characteristics even in the absence of knowledge of the etiology.
DEFINITION OF DIABETES MELLITUS
Diabetes mellitus is characterized by chronic hyperglycemia with disturbances of carbohydrate, fat, and protein metabolism resulting from defects in insulin secretion, insulin action, or both. When fully expressed, diabetes is characterized by fasting hyperglycemia, but the disease can also be recognized during less overt stages, most usually by the presence of glucose intolerance. The effects of diabetes mellitus include long-term damage, dysfunction, and failure of various organs, especially the eyes, kidneys, heart, and blood vessels. Diabetes may present with characteristic symptoms such as thirst, polyuria, blurring of vision, weight loss, and polyphagia, and in its most severe forms, with ketoacidosis or nonketotic hyperosmolarity, which, in the absence of effective treatment, leads to stupor, coma, and death. Often symptoms are not severe or may even be absent. Hyperglycemia sufficient to cause pathologic functional changes may quite often be present for a long time before the diagnosis is made. Consequently, diabetes often is discovered because of abnormal results from a routine blood or urine glucose test or because of the presence of a complication. In some instances diabetes may be apparent only intermittently, as, for example, with glucose intolerance in pregnancy or gestational diabetes, which may remit after parturition. In some individuals the likelihood of developing diabetes may be recognized even before any abnormalities of glucose tolerance are apparent. During the evolution of type 1 diabetes, for example, immunologic disturbances such as islet cell or other antibodies are present, and these may precede clinically apparent disease by months or even years (1). In some families it is possible to recognize certain gene mutations that are strongly associated with certain forms of diabetes, such as variations in the glucokinase gene or hepatic nuclear factor genes that cause youth or early adult-onset diabetes (2). These genetic abnormalities are detectable at any time.
Although a number of specific causes of diabetes mellitus have been identified, the etiology and pathogenesis of the more common types are less clearly understood. The majority of cases of diabetes fall into two broad etiopathogenetic categories, now called type 1 and type 2 diabetes (3,4), but the extent of heterogeneity among these types remains uncertain. Because of the increasing number of forms of diabetes for which a specific etiology can be recognized, the current clinical classification, proposed by the American Diabetes Association (ADA) in 1997 (3) and adopted by the World Health Organization (WHO)
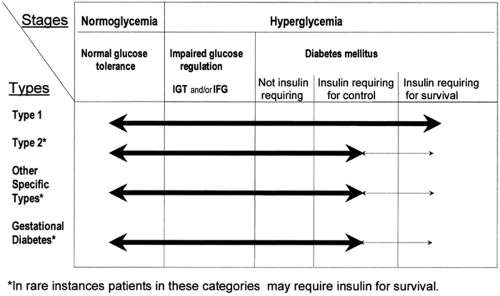
in 1999 (4) and that supersedes the previously internationally recognized 1985 WHO classification (5), now classifies diabetes according to both clinical stages and etiologic types (Fig. 19.1). The clinical staging reflects that diabetes progresses through several stages during its natural history and that individual subjects may move from one stage to another in either direction.
in 1999 (4) and that supersedes the previously internationally recognized 1985 WHO classification (5), now classifies diabetes according to both clinical stages and etiologic types (Fig. 19.1). The clinical staging reflects that diabetes progresses through several stages during its natural history and that individual subjects may move from one stage to another in either direction.
CLINICAL STAGES
Individuals who ultimately develop diabetes pass through several clinical stages during its development. Initially, glucose regulation is normal and no abnormality of glycemia can be identified even if these individuals undergo an oral glucose tolerance test (OGTT). This stage is followed by a period of variable duration in which glucose regulation is impaired. They may have some abnormality of the fasting glucose concentration, or if they receive an OGTT, they may demonstrate impaired glucose tolerance. Diabetes itself is characterized by either fasting glycemia or marked abnormalities of glucose tolerance, or both. Once diabetes develops, glycemia may be controlled by lifestyle changes such as diet and increased physical activity in some patients, whereas in others insulin or oral hypoglycemic agents are needed for its control or to prevent ketosis and ketoacidosis. If insulin is required to prevent ketosis, such patients are designated as “insulin requiring for survival.” In all forms of diabetes, there may be remission in the extent of hyperglycemia. Patients may revert to having impaired glucose regulation or even normal glycemia, particularly if diabetes is of recent onset. This is seen most frequently in patients with recent-onset type 2 diabetes, in whom lifestyle intervention and/or early aggressive treatment of the glycemia may result in apparent reversal of the abnormality with reversion to impaired or normal glucose tolerance (6). This may also be seen in type 1 diabetes, in which after a short period of insulin treatment, there may be a variable period when insulin is no longer required for survival and glucose tolerance may improve—the so-called honeymoon period. Eventually such patients do need insulin treatment for survival (7). Gestational diabetes often is followed by improved glucose tolerance following parturition, and for a variable period, such women may be normoglycemic. With a subsequent pregnancy, gestational diabetes is likely to recur. Many women who have had gestational diabetes develop diabetes within a few years when they are not pregnant; thus, even in the face of normal glycemia, such women can be recognized as being at high risk of developing type 2 diabetes (8).
All subjects with diabetes can be classified according to clinical stage regardless of the underlying etiology of the diabetes. The stage of glycemia may change over time, depending on the extent of the underlying disease process. The disease process may be present but may not have progressed far enough to cause clinically identifiable abnormalities of glucose metabolism. For example, antibodies to islet cells, insulin, or glutamic acid decarboxylase (GAD) in a normoglycemic individual indicate a high likelihood for ultimate progression to type 1 diabetes (9). There are few sensitive or specific early indicators of the likelihood for development of type 2 diabetes, but the disease process may be identified before the development of overt diabetes.
Impaired glucose regulation refers to the metabolic stage intermediate between normal glucose homeostasis and diabetes that can be identified by impaired glucose tolerance (IGT) or impaired fasting glucose (glycemia) (IFG) (3,4). IFG and IGT are not synonymous and may represent different abnormalities of glucose regulation, although they may occur together. Individuals with either of these states of impaired glucose regulation have a high risk of progressing to diabetes (10,11,12). IGT can be assessed only if OGTTs are carried out, whereas IFG refers to fasting glucose concentrations that are lower than those required for the diagnosis of diabetes but higher than those usually found in subjects with normal glucose tolerance. Subjects with IGT or IFG usually have normal or slightly elevated levels of glycosylated hemoglobin (13). IGT is frequently associated with the
presence of other indicators of the metabolic or insulin resistance syndrome (14).
presence of other indicators of the metabolic or insulin resistance syndrome (14).
ETIOLOGIC TYPES
The etiologic classification of diabetes mellitus currently recommended by WHO and the ADA is presented in Table 19.1. This classification differs considerably from the previously recommended classification, which used the terms insulin-dependent diabetes and non-insulin-dependent diabetes (5). These terms, however, were frequently misused and at best classified patients based on treatment needs rather than on etiologic characteristics. The terms type 1 and type 2 diabetes (with Arabic numerals) have been adopted for the most common forms of diabetes mellitus.
TABLE 19.1. Etiologic Classification of Disorders of Glycemia | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
TABLE 19.2. Other Specific Types of Diabetes Mellitus | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
TYPE 1 DIABETES MELLITUS
Type 1 diabetes is the form of the disease due primarily to β-cell destruction. This usually leads to a type of diabetes in which insulin is required for survival. Individuals with type 1 diabetes are metabolically normal before the disease is clinically manifest, but the process of β-cell destruction can be detected earlier by the presence of certain autoantibodies. Type 1 diabetes usually is characterized by the presence of anti-GAD, anti-islet cell, or anti-insulin antibodies, which reflects the autoimmune processes that have led to β-cell destruction. Individuals who have one of more of these antibodies can be subclassified as having type 1A, immune-mediated type 1 diabetes (3,4).
Particularly in nonwhites, type 1 diabetes can occur in the absence of autoimmune antibodies and without evidence of any autoimmune disorder. In this form of type 1 diabetes, the natural history also is one of progressive disease with marked hyperglycemia resulting in an insulin requirement for prevention of ketosis and survival. Such individuals are classified as having type 1B, or idiopathic, diabetes (15).
Type 1A diabetes shows strong associations with specific haplotypes or alleles at the DQ-A and DQ-B loci of the human leukocyte antigen (HLA) complex (16). The rate of β-cell destruction is quite variable, being rapid in some individuals, especially in infants and children, and slower in adults. Some have modest fasting hyperglycemia that can rapidly change to severe hyperglycemia or ketoacidosis, and others, particularly adults, may retain some residual β-cell function for many years and have sometimes been termed as having “latent autoimmune diabetes” (17,18). Such individuals may become dependent on insulin for survival only many years after the detection of diabetes. Individuals with type 1 diabetes have low or undetectable levels of insulin and plasma C-peptide. Patients with type 1A diabetes are also more likely to have other concomitant autoimmune disorders, such as Graves disease, Hashimoto thyroiditis, Addison disease, vitiligo, or pernicious anemia.
Type 1B, or idiopathic, diabetes is characterized by low insulin and C-peptide levels similar to those in type 1A. Such patients are prone to ketoacidosis, although they have no clinical evidence of autoimmune antibodies. Many of these patients are of African or Asian origin. They may suffer from episodic ketoacidosis, but the pathogenetic basis for their insulinopenia remains obscure.
TYPE 2 DIABETES MELLITUS
Type 2 diabetes is the most common form of diabetes. It is characterized by disorders of insulin action and insulin secretion, either of which may be the predominant feature. Usually, both are present at the time diabetes becomes clinically manifest. Although the specific etiology of this form of diabetes is not known, autoimmune destruction of the β-cells does not occur.
Patients with type 2 diabetes usually have insulin resistance and relative, rather than absolute, insulin deficiency. At the time of diagnosis of diabetes, and often throughout their lifetimes, these patients do not need insulin treatment to survive, although ultimately many require it for glycemic control. This form of diabetes is associated with progressive β-cell failure with increasing duration of diabetes (19). Ketoacidosis seldom occurs spontaneously but can arise with stress associated with another illness such as infection.
Most patients with type 2 diabetes are obese when they develop diabetes, and obesity aggravates the insulin resistance. Type 2 diabetes frequently goes undiagnosed for many years because the hyperglycemia develops gradually and in the earlier stages is not severe enough to produce the classic symptoms of diabetes; however, such patients are at increased risk of developing macrovascular and microvascular complications. Their circulating insulin levels may be normal or elevated yet insufficient to control blood glucose levels within the normal range because of their insulin resistance. Thus, they have relative, rather than absolute, insulinopenia. Insulin resistance may improve with weight reduction or pharmacologic treatment and results in normalization of their glycemia. Type 2 diabetes is seen frequently in women who have a previous history of gestational diabetes and in individuals with other characteristics of the insulin resistance syndrome, such as hypertension or dyslipidemia.
Patients who are not obese and who have relatives with type 1 diabetes, especially those of European origin, may present with a clinical picture consistent with type 2 diabetes but may have autoantibodies similar to those found in type 1 diabetes. Such patients have type 1A diabetes yet may appear to have type 2 diabetes unless antibody determinations are made.
The risk of developing type 2 diabetes increases with age, obesity, and physical inactivity. Type 2 diabetes shows strong familial aggregation, so that persons with a parent or sibling with the disease are at increased risk, as are individuals with obesity, hypertension, or dyslipidemia and women with a history of gestational diabetes. The frequency of type 2 diabetes varies considerably among different racial or ethnic subgroups. Persons of Native American, Polynesian or Micronesian, Asian-Indian,
Hispanic, or African-American descent are at higher risk than persons of European origin (20). Although the disease is most commonly seen in adults, the age of onset tends to be earlier in persons of non-European origin. The disease can occur at any age and is now seen in children and adolescents (21,22,23,24).
Hispanic, or African-American descent are at higher risk than persons of European origin (20). Although the disease is most commonly seen in adults, the age of onset tends to be earlier in persons of non-European origin. The disease can occur at any age and is now seen in children and adolescents (21,22,23,24).
OTHER SPECIFIC TYPES OF DIABETES
Other specific types of diabetes are those in which the underlying defect or disease process can be identified in a relatively specific way or those that have other distinctive, distinguishing features. This category encompasses a variety of types of diabetes secondary to other specific conditions or associated with particular diseases or syndromes with a distinct etiology.
The categories and many of the causes of other specific types of diabetes are shown in Table 19.2. These include genetic defects of β-cell function, which encompass several types of diabetes that are associated with specific monogenic defects. Most of these are characterized by a dominant pattern of inheritance and the onset of hyperglycemia at an early age. They are often referred to as maturity-onset diabetes of the young (MODY). They are characterized by impaired insulin secretion with minimal or no defects in insulin action. They are inherited in an autosomal dominant pattern but are heterogeneous. A number of specific genetic defects have been identified, including variations in hepatic nuclear factor 4α (HNF4α) (MODY1), glucokinase (MODY2), HNF1α (MODY3), insulin-promoting factor 1 (IPF1) (MODY4), and HNF3β genes (MODY5) (2). There are also some forms of MODY for which the genetic defect remains to be identified. Another form of autosomal dominant diabetes is due to a mutation in the KATP channel subunit (SUR1) of the sulfonylurea receptor that gives rise to congenital hyperinsulimemia and loss of insulin secretory capacity in young adults, leading to impairment of glucose tolerance and diabetes in middle age (25).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree