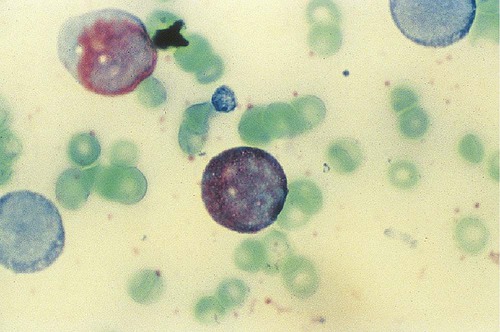
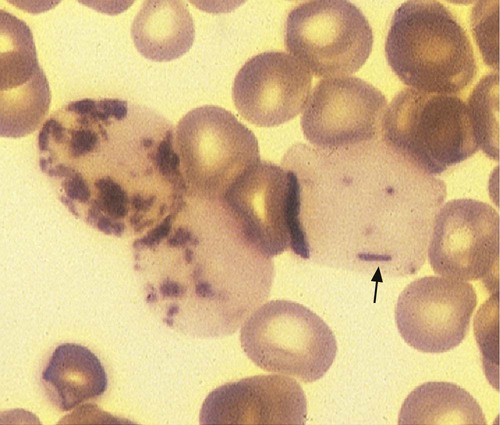
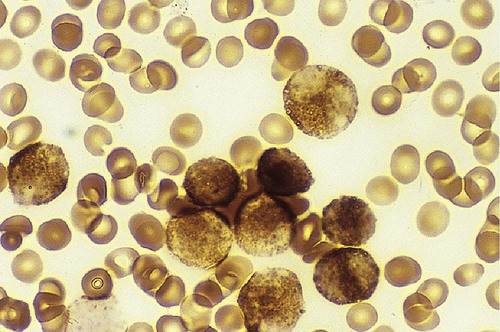
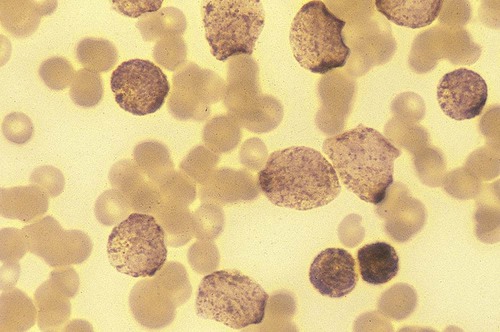
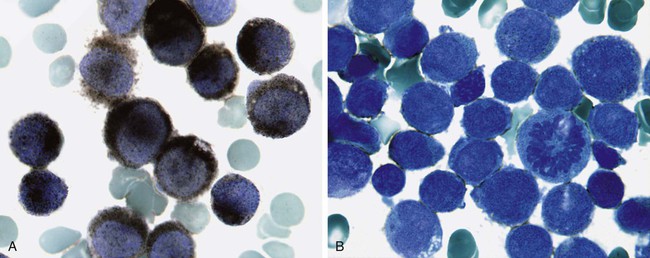
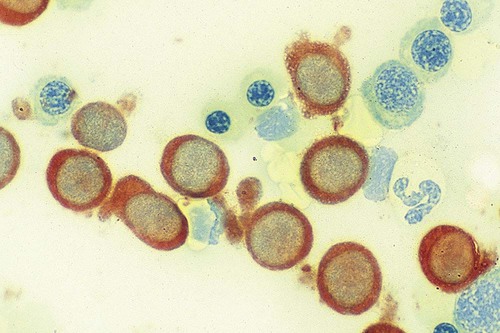
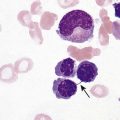
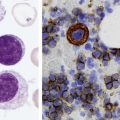
After completion of this chapter, the reader will be able to: 1. Discuss the purpose of performing cytochemical stains. 2. Determine appropriate specimen types, handling procedures, and fixatives for cytochemical stains, considering length of stability of specimens if staining is delayed. 3. Discuss the principles and cell staining patterns for the following tests: myeloperoxidase, Sudan black B, esterases, periodic acid–Schiff, leukocyte alkaline phosphatase, and tartrate-resistant leukocyte acid phosphatase. 4. Name the cell stage that is evaluated using the stains listed in item 3. 5. Interpret the results of the stains listed in item 3. 6. Discuss the selection of controls in cytochemical staining. 7. Describe routine troubleshooting in cytochemical techniques. Cytochemistry is the study of the chemical constituents of cells. These elements may be enzymatic (e.g., peroxidase) or nonenzymatic (e.g., lipids and glycogen). The cellular morphology of peripheral blood and bone marrow often provides a provisional diagnosis, and since the early twentieth century, cytochemical staining of cells has provided a useful adjunct for differentiation of hematopoietic diseases, especially acute leukemias. Immunophenotyping, high-resolution cytogenetic studies, and molecular genetic analysis have reduced the importance of cytochemical testing. Microarray technology can quantify the expression of thousands of genes in a single analysis.1,2 However, cytochemical testing still has limited use in many laboratories, especially in equivocal cases or where more sophisticated technology is not available.3,4 (Leukemias are discussed in Chapters 29, 36, and 37; cytogenetics, in Chapter 31; molecular diagnostics, in Chapter 32; and flow cytometry, in Chapter 33.) Myeloperoxidase (MPO) (Figures 30-1 and 30-2) is an enzyme found in the primary granules of neutrophils, eosinophils, and, to a certain extent, monocytes. Lymphocytes do not exhibit MPO activity. This stain is useful for differentiating the blasts of acute myeloid leukemia (AML) from those of acute lymphoblastic leukemia (ALL). When hydrogen peroxide is present, MPO oxidizes dye substrates, creating black to red-brown staining (depending on the substrate) at the site of the activity. Benzidine had been the substrate most often used, but because of its carcinogenic properties, other substrates such as 3,3′-diaminobenzidine or p-phenylenediamine dihydrochloride and catechol are currently used.5–7 SBB staining (Figure 30-3) is another useful technique for the differentiation of AML from ALL. The staining pattern is quite similar to that of MPO; SBB staining is possibly a little more sensitive for the early myeloid cells. Granulocytes (neutrophils) show a positive reaction to SBB from the myeloblast through the maturation series. The staining becomes more intense as the cell matures as a result of the increase in the numbers of primary and secondary granules. Monocytic cells can demonstrate negative to weakly positive staining, showing diffuse activity. Lymphoid cells generally do not stain. In ALL, fewer than 3% of the blast cells show a positive reaction.8–10 Esterase stains can be used to distinguish acute leukemias that are granulocytic from leukemias that are primarily of monocytic origin. When naphthol AS-D chloroacetate is used as a substrate, the reaction is positive in the granulocytic cells and negative to weak in the monocytic cells (Figure 30-4). Chloroacetate esterase is present in the primary granules of neutrophils. Leukemic myeloblasts generally show a positive reaction. Auer rods show positivity as well. α-Naphthyl acetate, in contrast to naphthol AS-D chloroacetate, reveals strong esterase activity in monocytes that can be inhibited with the addition of sodium fluoride.8,13 Granulocytes and lymphoid cells generally show a negative result on nonspecific esterase staining (Figure 30-5). A positive α-naphthyl butyrate esterase reaction is also seen in monocytes. α-Naphthyl butyrate is less sensitive than α-naphthyl acetate, but is more specific. Granulocytes and lymphoid cells generally show a negative reaction (Figure 30-6). In myelomonocytic leukemia, positive AS-D chloroacetate activity and positive α-naphthyl butyrate or α-naphthyl acetate activity should be seen because myeloid and monocytic cells are present. In myelomonocytic leukemia, at least 20% of the cells must show monocytic differentiation that is nonspecific esterase positive and is inhibited by sodium fluoride. In the pure monocytic leukemias, 80% or more of the blasts are nonspecific esterase positive and specific esterase negative.
Cytochemistry
Introduction and Principle
Stains and Interpretations
Myeloperoxidase
Principle
Sudan Black B
Interpretation
Esterases
Interpretation