Mutations in KIT are found in exons 9, 11, 13, and 17, and there is no one predominant point mutation.6 Because of this, molecular testing for KIT mutations must evaluate multiple regions of the gene by extended sequencing or multiplexed polymerase chain reaction (PCR) tests. Emerging evidence suggests that not all KIT mutations activate its function, resulting in some mutations being insensitive to KIT inhibitors used for patient treatment.16 NRAS mutations cluster in the RAS hotspot mutation site Q61, usually Q61L and less frequently Q61R and Q61H. NRAS mutations are more frequent in older individuals and are equally common in melanomas arising from the skin with chronic or intermittent sun damage.2,17 The vast majority of BRAF mutations in melanoma involve a substitution for valine at the 600th amino acid position to glutamine (V600E).5,18 The frequency of the BRAF V600E mutation is inversely correlated with age since it is most frequent in melanomas that appear on the skin without chronic sun damage in young adults. BRAF V600K is the second most common variant, which increases in incidence with age. V600D or V600R mutations are much less frequent.8 Overall, up to 90 different point mutations have been described in BRAF in different cancers, some of which are activating and others are inhibiting its enzymatic activity.18
Aberrations in the phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin pathway, including the phosphatase and tensin homolog (PTEN), are also noted in a significant number of melanomas, but these do not seem to function as true drivers of the malignant phenotype. PTEN alterations include missense mutations, deletions, and insertions, as well as loss of heterozygosity and epigenetic silencing, making interrogation for mutations and genomic rearrangements in PTEN necessary.19,20 The pathogenesis of melanoma, like most other cancers, requires the presence of a driver oncogene and the dysregulation of cell cycle control and apoptosis to provide the full oncogenic signaling and ability to grow autonomously. These happen with the frequent mutations or genetic deletions of CDKN2A, cyclin D1, or the amplification cyclin-dependent kinase 4.21
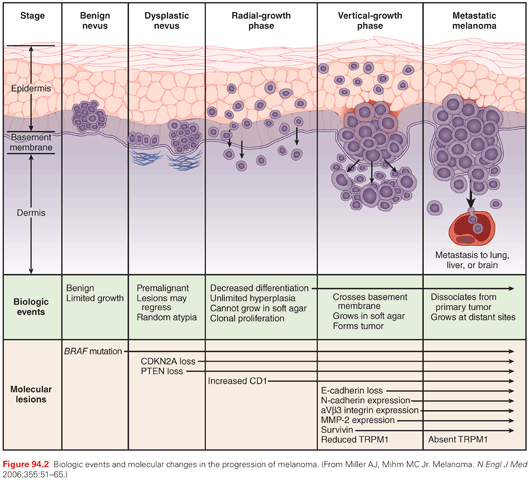
Progression of Melanocytes to Cutaneous Melanoma
Genetic Events in Melanocyte to Melanoma Progression and Oncogene-Induced Senescence
BRAF and NRAS are founding mutations of cutaneous melanoma that are frequently present in benign nevi.22 Despite of the presence BRAF and NRAS mutations, nevi have an exceedingly low proliferative activity and infrequently progress to melanoma. This is explained because of the phenomenon of oncogene-induced senescence preventing malignant progression to melanoma, where these mutations require functioning with additional genetic events that lead to dysregulation of cell cycle control to result in the development of a progressive melanoma.15,23 The model for oncogene-induced senescence in melanoma is based on the identity of the main driver mutations (BRAF and RAS) in nevi, the initial phase of proliferative activity they spark, the formation of a benign nevus in association with the induction of senescence markers (cell cycle arrest, induction of the tumor suppressor p16INK4a, endoplastic reticulum stress markers and increased SA-βGal activity, and possibly additional senescence biomarkers), and the subsequent cessation of expansion, which is typically maintained for decades.24
Cellular Changes in Melanocyte to Melanoma Progression
The transition from melanocyte to metastatic melanoma involves several histologic intermediates, including melanocytic atypia, atypical melanocytic hyperplasia, radial growth phase melanoma, vertical growth phase melanoma, and metastatic melanoma. Atypical melanocytes arising in a preexisting nevus or de novo are very common but rarely progress to melanoma. However, some patients develop confluent atypical melanocytic hyperplasia at the dermal/epidermal junction or nests of atypical melanocytes in the epidermis or at the dermal/epidermal junction. As this process progresses, it reaches a point at which a diagnosis of melanoma is warranted.
Early cutaneous melanomas usually proceed to grow radially, and this is called the radial growth phase (RGP) of melanoma, which may continue for years before progressing to the vertical growth phase (VGP) (Figs. 94.2 and 94.3). The RGP of a cutaneous melanoma may include either melanoma in situ or superficial invasion into the papillary dermis, or both. Melanomas in RGP present clinically as enlarging macules or very minimally raised papular lesions, which are typically (but not always) pigmented. These lesions are rarely symptomatic. If not recognized, these lesions typically progress to the VGP, manifest clinically by a nodular growth of the lesion, often described by the patient as a lesion that began to “raise up.” This vertical growth usually arises as a nodule within the RGP component and encompassing only part of the RGP (see Fig. 94.3A,C). Thus, the VGP appears to represent further steps in the process of malignant transformation due to clonal changes in the cells of the RGP.
Some melanomas present as metastatic melanoma in lymph nodes, skin, subcutaneous tissue, or visceral sites without an apparent primary cutaneous site. In some cases, these have been associated with a history of a regressed primary melanocytic lesion. In other cases, such an explanation is less clear. In all of these cases, the prospect of early diagnosis of melanoma is compromised, and the risk of melanoma-associated mortality is increased.
Malignant melanoma is the sixth most common US cancer diagnosis. The actual incidence of melanoma is increasing more rapidly than that of any other malignancy. It was estimated that 76,690 men and women (45,060 men and 31,630 women) will be diagnosed with and 9,480 men and women will die of melanoma of the skin in 2013.25 This amounts to 4% of new cancer diagnoses and 1.5% of cancer deaths. In the early part of the 20th century, the lifetime risk of a white person developing melanoma was approximately 1 in 1,500. Currently, 1 in 49 men and women will be diagnosed with melanoma of the skin during their lifetime. Its incidence is second only to breast cancer for women from birth to age 39 years; similarly, it is the second most common cancer diagnosis for men through age 39 years, slightly less common than leukemia.26 Overall 5-year survival rates for melanoma have increased from 82% in the late 1970s (1975 to 1977) to 91% in the more recent era (2002 to 2006).26
This is a disease that disproportionately affects whites over African Americans, Asians, or Hispanics. In the United States, whites account for 98.2% of cutaneous melanomas reported in the National Cancer Database, with African Americans accounting for 0.7% and Hispanics accounting for 1.1%.1 This is best explained by a combined effect of UV sunlight exposure and fair skin. It is most striking that the highest per capita incidence of melanoma worldwide is in Australia, and that this high incidence afflicts primarily the Australians of Western European descent who have fair skin, and not the darker-skinned aboriginal population. It is also notable that these fair-skinned European descendants who moved to Australia have much higher incidences of melanoma than the Western European populations that remain in the higher latitudes of Europe. In migrant populations, individuals who move during childhood to areas with greater sun exposure develop melanoma at rates higher than those of their country of origin and similar to those of their adopted country.27
In nonwhite populations, there is a much higher proportion of melanomas in acral (subungual, palmar, plantar) and mucosal locations. However, the incidences of those types of melanoma are similar across races. Their higher relative proportion in Asians and African Americans can be best explained by the disproportionate increase in nonacral cutaneous melanomas in fair-skinned whites rather than by an absolute increase in risk of acral and mucosal melanomas in nonwhite populations.
Ocular and nonacral cutaneous melanomas are 50- to 200-fold more likely in white populations than in nonwhite populations, but melanomas in acral and mucosal sites are within twofold of each other across racial groups. Similarly, the increased incidence of melanoma over the last few decades can be explained primarily by increased incidence in white populations, not in nonwhite populations.28 These observations support the hypothesis that most cutaneous melanomas in white populations are etiologically related to sun exposure but that there may be a baseline risk of melanoma in other locations that is unrelated to sun damage. There are significant molecular differences between acral melanomas and melanomas arising on the skin associated with chronic sun damage, with B-RAF and N-RAS mutations in approximately 80% of melanomas on chronically sun-damaged skin, whereas those mutations were uncommon in melanomas from acral or mucosal sites or from skin without chronic sun damage.2
Data from the Surveillance, Epidemiology, and End Results program reveal an increase in age-adjusted melanoma incidence rates from 8.2 per 100,000 in the 1970s (1974 to 1978) to 18.7 per 100,000 in more recent years (1999 to 2003).29 From 1990 to 2003, during which there was a 16% decrease in male cancer deaths overall for all cancers, there was a 2% increase in mortality rate from melanoma. From 1991 to 2003, during which there was an 8% decrease in cancer deaths overall for women, there was only a 4% decrease in mortality rate associated with melanoma.26
In Australia, and to a lesser extent in the United States, there has been a substantial increase in awareness about melanoma and the value of screening by total-body skin examinations. There also has been a greater proportion of patients diagnosed at earlier and noninvasive stages of disease. Thus, part of the increase in incidence may be explained by increased early diagnosis of lesions with low metastatic potential. However, there has also been a significant increase in mortality from melanoma over the last few decades.29
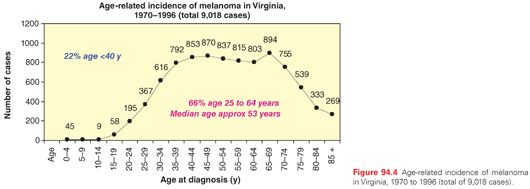
In the United States and Australia, the gender ratio of melanoma at diagnosis is 2 male to 1 female, but it depends on the age group. Analysis of incidence data for invasive melanoma diagnosed from 1992 to 2006 from 12 cancer registries that participate in the Surveillance, Epidemiology, and End Results program of the National Cancer Institute revealed that, by age, the men-to-women rate ratio ranged from 1.3 (95% confidence interval [CI], 1.2 to 1.3) for ages 40 to 64 years for incidence to 2.6 (2.5 to 2.7) for older than 65 years for both incidence and mortality. However, between the age of 15 and 39 years old, melanoma is more common in females (rate ratio = 0.6).25 The median age of melanoma patients has increased from 51 years in the 1970s (1974 to 1978) to 57 years in a more recent time period (1999 to 2003). Nonetheless, the median age for diagnosis of melanoma is approximately 10 years lower than the current median age of diagnosis for the more common solid tumors, such as colon, lung, or prostate cancer. The large majority (approximately 80%) of patients with melanoma are diagnosed in the productive years from age 25 to 65 as shown for a representative population from the state of Virginia (Fig. 94.4). Melanoma is common in patients in their 20s and older, but it also is observed in teenagers, and occasionally even in infants and neonates. For women aged 25 to 35 years, melanoma is the leading cause of cancer-related death.
MELANOMA IN CHILDREN, INFANTS, AND NEONATES
Diagnosis and management of melanoma in children, infants, and neonates is complicated by several factors: (1) excisional biopsy of skin lesions often is not feasible under local anesthesia in young children, and (2) pigmented skin lesions with substantial cellular atypia but with structural symmetry may be Spitz nevi, which typically have benign behavior. Thus, some young patients with changing pigmented skin lesions are observed longer than would be advisable because biopsy is more problematic than in most adults. In addition, young patients may undergo incomplete shave biopsy to avoid a full-thickness excision, and information is lost about the architecture of the lesion, leaving a diagnostic dilemma between melanoma and Spitz nevus. Even in the best of circumstances, some melanocytic tumors are difficult to diagnose with certainty. This has led to a formal definition of melanocytic tumors of uncertain malignant potential.30
Melanoma deaths in children and young adults have a large effect on total years of life lost because of melanoma. Current recommendations for management of melanoma in children and infants are the same as for adults, and outcomes are generally believed to be comparable.31
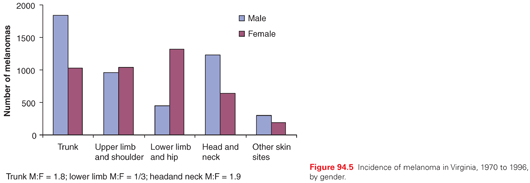
Cutaneous melanoma can occur at any skin site in the body. The most common sites in males are on the back and in the head and neck regions. In women, the most common sites are in the lower extremities, commonly below the knee (Fig. 94.5). Lentigo maligna melanoma (LMM) most commonly arises on sun-damaged surfaces of the head and neck in older patients. Acral lentiginous melanoma (ALM) is most common on subungual and other acral locations.
Ultraviolet Light Exposure
The demographic features of cutaneous melanoma have implicated UV light exposure as a major etiologic factor in the development of melanoma. Multiple studies continue to support an etiologic association between UV irradiation and melanoma.32 UVC radiation is generally absorbed by the ozone layer. UVB radiation (290 to 320 nm) is associated with sunburn and induction of tanning by melanin pigment production. There are substantial data to support its etiologic role in melanoma.32 There is also some evidence implicating UVA radiation (320 to 400 nm), although UVA is more associated with chronic sun damage changes.33 However, the relative role of each type of UV irradiation in melanoma etiology is debated. Animal data suggests that sun exposure early in life increases the risk of melanoma. Human skin grafted on mice will develop nevi and melanomas in the presence of UVB irradiation, further supporting the role of UVB irradiation in melanoma.34 Similar to the animal modeling, sunburns early in life have been implicated in melanoma incidence.35 However, chronic sun exposure in individuals who tan well may even be protected against melanoma. The role of sunlight intensity and frequency is debated, but both chronic and intermittent exposure may be relevant.32 Current data suggest that UV radiation causes melanoma by a combination of DNA damage, inflammation, and immune suppression.36
Tanning bed use has been implicated in the etiology of melanoma, in particular tanning bed use in adolescence or early adulthood.37 Tanning bed use has been formally classified as a carcinogen, and increased awareness of the harmful effects of UV exposure promise to control the increase in melanoma incidence.
Physical Traits
Several physical traits have been linked to increased incidence of cutaneous melanoma. These include blond or red hair, green or blue eyes, presence of multiple (>100) melanocytic nevi, and five or more atypical nevi. A prior diagnosis of melanoma is associated with an eight-fold increased risk of developing a secondary melanoma.
Familial Predisposition
It has been estimated that 5% of melanomas occur in high-risk families with an autosomal dominant inheritance with incomplete penetrance.38 The most frequent and highest penetrance melanoma susceptibility gene is a germline mutation in CDKN2A, a tumor suppressor gene that encodes for two different proteins, p16INK4A and p14 ARF.39 These proteins control cell cycle progression and apoptosis, and have roles in correcting DNA damage and cellular senescence. CDKN2A mutations have been reported in approximately 25% of melanoma-prone families, but this frequency varies highly on the selection criteria used and the region of the world where it is studied. The rare autosomal dominant inherited familial atypical multiple mole melanoma-pancreatic cancer syndrome is associated with CDKN2A mutations, and less frequently to BRCA2 mutations.40 Another germline mutation linked to familial melanoma is cyclin-dependent kinase 4, which is linked to the function and p16INK4A and controls the retinoblastoma pathway. A germline mutation in microphthalmia-associated transcription factor (MITF E318K) represents a medium-penetrance susceptibility gene predisposing to familial melanoma, as well as to sporadic melanoma and renal cell carcinoma.41,42 The E318K mutation in MITF disrupts sumoylation and enhances transcription of MITF-responsive genes. Other common risk factors include dysplastic nevus syndrome, xeroderma pigmentosum, and a family history of melanoma even without the known genetic traits. The association of melanoma with Li-Fraumeni syndrome, with germline mutations in p53, is currently unclear.43
Pregnancy and Estrogen Use
Older literature suggested anecdotally that the incidence of melanoma was higher in pregnant females and that they had a particularly bad outcome. However, multiple systematic and larger studies have shown no evidence of any negative (or positive) impact of prior, concurrent, or subsequent pregnancy on clinical outcome.44,45 Similarly, there is no clear prognostic relevance for birth control pills or estrogen replacement therapy.46 The prior sense of an apparent association of pregnancy and melanoma may be due to melanoma being the second most frequent cancer in females of childbearing age. The general recommendation for treatment of women with melanoma diagnosed during pregnancy is to manage them in the same fashion as patients who are not pregnant. Depending on the time during pregnancy at which a melanoma is diagnosed, there can be circumstances in which radiologic imaging may be limited because of concern for the fetus, and major surgery may be delayed until the fetus is at an age when it can survive independently. However, the excision of a primary melanoma certainly can be done in almost any circumstance, under local anesthesia.
The other related question often asked by patients is whether it is advisable to become pregnant and to bear a child after treatment for melanoma. As just stated, there is no evidence that a subsequent pregnancy adversely impacts outcome. However, the more interesting and challenging question is the more personal or social issue of the potential for premature parental death due to melanoma. Thus, it is helpful for patients to understand their risk of future recurrence and melanoma-related mortality because that translates into the risk that the child will grow up losing a parent. Measures of the risk of future disease progression can be defined based on the initial prognosis and the subsequent elapsed time without recurrence, and such information may help to guide patients with this challenging question.47
Melanomas diagnosed and treated during the RGP have an excellent prognosis. Thus, prevention and early diagnosis can have a great impact on decreasing melanoma morbidity and mortality. The apparent leveling off of melanoma-related mortality rates in Australia and the United States likely is the result of better screening and prevention.
Sun Protection
UV exposure and sunburns, in particular, appear to be etiologic in most melanomas. Thus, protection from UV light, especially in fair-skinned individuals, is believed to have substantial benefit in preventing melanoma.
A clinical trial has provided evidence that regular sunscreen use helps prevent melanoma.48 This was a randomized trial from March 1992 to August 1996 of 1,621 randomly selected adult residents of a Queensland township in Australia with an initial primary end point testing the prevention of squamous cell and basal cell carcinomas, which the study did demonstrate.49 Prevention of the development of melanoma was a prespecified secondary end point. Participants were randomly assigned to either a planned sunscreen intervention group or a control group using sunscreen at their discretion. The intervention group received broad-spectrum, sun protection factor (SPF) 16 sunscreen every morning, and was instructed to reapply the sunscreen after a long sun exposure, heavy sweating, or after bathing. After a 10-year follow-up, regular sunscreen use decreased by half the rate of developing new melanomas. This conclusion was based on 11 participants in the intervention group and 22 in the control group being newly diagnosed with either invasive or in situ melanoma (p = 0.051). The incidence of invasive melanoma decreased by 73% in the intervention group compared with the control group (3 versus 11 patients, respectively; p = 0.045). Therefore, this study provides evidence that use of sunscreen can decrease the incidence of melanoma development.
There are limitations inherent in sunscreen use as the primary means to protecting from UV light damage. One is that certain body sites are not easily covered with sunscreen, such as the scalp. More important, even “waterproof” sunscreens wash off or become less effective with time. Most people also forget to reapply sunscreens frequently enough and may still get burns. There are also sociologic issues, which may differ for different populations and are arguable. However, it is worth considering the provocative findings of a study performed on young adults from Western Europe, who were randomized to receive either SPF10 or SPF30 sunscreen. In a blinded fashion, they were asked to report sun exposure times and sunburns. The number of sunburns was the same in both groups, and sun exposure was greater in the SPF30 group, suggesting that some populations may stay in the sun until they get a burn, and that sunscreen simply helps them to stay in the sun longer.50 The sun-seeking behavior has been related to an evolutionary need that favored UV exposure to make vitamin D in the skin in populations that migrated to areas of the world with lower sun exposure. In mouse models, the exposure to UV light was linked to increased production of beta-endorphins and recurrent seeking of UV exposure.51
It is safe to say that the best protection from the sun is a building, the next best is protective clothing, and the third best is sunscreen. Patients should be advised to use all three. Avoiding midday sun from about 11 a.m. to 3 p.m. by staying indoors is advised, as well as wearing clothing with a thick enough weave that it blocks sunlight, or a formal SPF rating, when possible. Hats are particularly helpful for the face and scalp, which often are highly exposed to sunlight and not so readily covered fully with sunscreen. Otherwise, sunscreen can provide protection to sun-exposed areas when outside.
Screening for Early Diagnosis
Self-Examination
For many patients, they, their spouses, or other family members may be able to screen effectively for new suspicious skin lesions, and this should be encouraged. It is more common for women to detect melanomas than for men to do so, either for themselves or for their partners. In any case, there is value in educating patients about how to detect melanomas if they are at high risk. As many as half of melanomas are identified by the patient or family,52 and patient self-examination has been associated with diagnosis of thinner melanomas.53 Teaching aids for patients on how to perform skin self-examination are available from the American Cancer Society and the American Academy of Dermatology. Patients with melanoma or at high risk should be seen regularly by a dermatologist. It is reasonable to suggest that patients perform skin self-examinations more often than their dermatology visits, although there are no proven guidelines. Doing a self-examination once a month may be the easiest for the patient to remember.
The role of skin cancer screening to decrease incidence and mortality from cutaneous melanoma has been prospectively studied in the Schleswig-Holstein project.54 This was an observational study comparing trends in melanoma mortality in a population-based skin cancer screening project conducted in the northern German region of Schleswig-Holstein, compared to neighboring regions in Germany and Denmark where no such screening was conducted. From July 1, 2003, to June 30, 2004, 360,288 individuals aged 20 years were screened by whole-body examination. They reported that mortality in Schleswig-Holstein melanoma declined by 48% when analyzed using log-linear regression to assess mortality trends. No such change in melanoma mortality rates was noted in the studied adjacent regions. This study provides strong evidence that skin cancer screening programs may reduce melanoma mortality.54
Management of the Patient with Numerous Atypical Moles
Some patients have numerous atypical moles. This presentation is commonly described as atypical mole syndrome, dysplastic nevus syndrome, or B-K mole syndrome.55 These patients have a heightened risk of melanoma, and this is commonly a familial feature. When associated with a family history of melanoma, patients with dysplastic nevus syndrome have a risk of melanoma that may approach 100%. These patients deserve particular attention to melanoma prevention through sun protection and to early diagnosis through aggressive screening. However, the optimal approach for screening is not defined. At a minimum, routine skin examinations by a dermatologist are usually recommended, as often as every 3 months. Visual inspection of the atypical nevi may be augmented by routine digital photography to facilitate detection of subtle changes in radial growth or other changes over time. Although these approaches commonly permit identification of melanomas when they are in situ or thin, it is not known whether they improve survival. In addition, concern remains that visual inspection alone, even for very experienced dermatologists, is inadequate to diagnose all melanomas when they are still curable. Thus, substantial effort is in progress to develop more sensitive and specific diagnostic tools than visual inspection alone. One that is employed routinely in many practices is dermoscopy, also known as epiluminescent microscopy. This involves use of a handheld microscope at the bedside to examine skin lesions in an oil immersion setting. This appears to improve diagnostic accuracy in experienced hands, and increasing experience has made its use more feasible in general practice, especially with considerations for standardization.56,57 When coupled with the use of a digital camera, the images can be stored and compared over time as well. Computer-assisted digital analysis of these images is also being studied but remains investigational.
Evaluation and management of patients with dysplastic nevus syndrome is complicated by the fact that very few dysplastic nevi will develop into melanoma. Estimates range from a risk of 1 per 1,000 nevi examined in a pigmented lesion clinic being melanoma to 1 per 10,000 nevi becoming melanoma per year.56,57 Recommendations for management of dysplastic nevi include those from the Melanoma Working Group in the Netherlands and by a National Institutes of Health Consensus Conference.58
It is tempting to consider excision of all dysplastic nevi. Although that remains an option, there is no proof that this will decrease risk. Melanomas may arise de novo in 30% to 70% of cases, and so it is not clear that removal of all suspicious nevi will lead to a meaningful improvement in survival. However, it is certainly appropriate to biopsy any nevus that is suspicious, especially one that is changing.
Testing for Genomic Changes in Melanoma
Understanding the genetic makeup of melanoma has become the cornerstone of advances in the management of advanced disease, and it is likely to have an increasing role in the management of earlier stage melanoma. Genetic analyses can be focused on driver oncogenic events or can provide a broader understanding of the genomic aberrations in the cancer. Their study is becoming a standard of care practice in melanoma.
Commercially available tests identifying the BRAF V600E/K mutation have been approved by the U.S. Food and Drug Administration (FDA) and other regulatory bodies as companion diagnostics for the use of novel BRAF and MEK inhibitors. These assays are frequently based on specific PCR probes labeled with fluorescent tags that bind to wild-type and V600E or V600K mutant BRAF sequences. These assays are performed in sections of formalin-fixed paraffin embedded tissue blocks routinely used for pathologic analyses. As with all techniques used to detect somatic mutations, they are limited by the amount of mutant sequence in the initial sample as well as DNA integrity in the sample, and their ability to detect non-V600E BRAF mutations since the primers used are usually restricted to this particular BRAF mutation. Multiplexed single nucleotide extension assays (i.e., Sequenom [San Diego, CA] or SNaPshot [Vanderbilt-Ingram Cancer Center, Nashville, TN]) evaluate a list of specific base mutations of interest, but do not identify mutations outside the interrogated bases. These techniques are designed for simultaneous interrogation of different point mutations. They are particularly suited for the targeted interrogation of known oncogenes that contain mutation hotspots, such as NRAS, BRAF, and GNAQ/GNA11, being relevant for melanoma.59
Traditional Sanger sequencing has been the gold standard for the detection of point mutations, but it has been shown to have lower sensitivity than the PCR-based assays, thereby leading to a higher frequency of false negative results. Pyrosequencing provides information from the sequencing 300 and 500 nucleotides at a time, resulting useful for the analysis of mutations clustered in a small gene region. It is a highly sensitive technique, being able to detect mutant DNA when only 5% of the total sample is from the cancer tissue.
Copy number analyses have been very useful in the discovery and description of genes and pathways involved in melanoma pathogenesis. Initially, probe sequences were derived from bacterial artificial chromosomes using array comparative genomic hybridization. More recently, single nucleotide polymorphism–based arrays have been introduced. But currently the approach of choice for the analysis of the DNA alterations in melanoma at the genome level is massively parallel sequencing techniques. These techniques enable the sequencing of exomes and entire genomes of tumor samples (compared to normal DNA from the same patient), with the simultaneous sequencing of a large number of genes and determination of mutations, genetic alterations, and copy number changes. The price and complexity of this type of analysis has rapidly improved, making it feasible to use beyond research studies. Limited panels performing next generation sequencing in what have been called “actionable” genes have been implemented for clinical use. These provide information based on sequencing data of 200 or so genes for which the available literature suggests that they could provide information which may be interpretable to decide on treatment options, in particular in terms of clinical trial participation with new targeted agents.60
A clinically applicable approach to genetic testing of melanomas is first performing a targeted testing for the mutation status of BRAF, NRAS, and KIT in cutaneous and mucosal melanoma samples before pursuing alternative mutation interrogation with higher throughput approaches. Next-generation sequencing may be applicable in situations where known mutations are not identified, and the identification of additional genetic mutations is needed.
Characteristics of Primary Melanoma
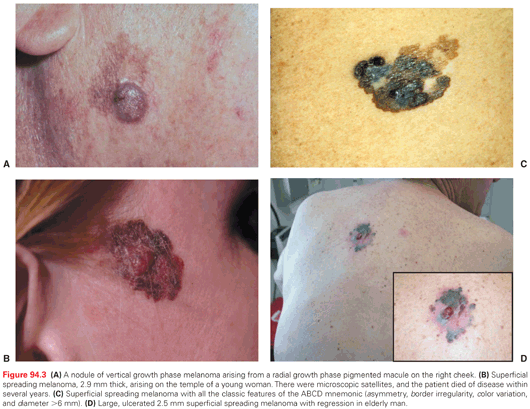
The classic appearance of primary cutaneous melanoma is summarized by the mnemonic ABCD for asymmetry, border irregularity, color variation, and diameter >6 mm (see Fig. 94.3). Because melanomas arise from melanocytes, which contain the melanin-synthetic pathway, melanomas classically are distinguished by their pigmentation. Melanomas may have shades of brown, black, blue, red, and white. However, there is a wide range in the appearance of melanomas. Some melanomas are pitch black. Others are shades of brown. Some have no visible pigment and appear skin-colored. Still others have a red color only. When melanomas have all of the classic ABCD features, they are typically easy to diagnose. However, those melanomas that lack some of these features can be difficult to diagnose. In addition, in patients with large numbers of atypical nevi, which may also have ABCD features, this mnemonic is often inadequate to aid in early diagnosis. The other important findings that may aid in early diagnosis are a change in a lesion over time or new development of a skin lesion. These warrant evaluation, and in high-risk patients there should be a low threshold for biopsy. In addition, some dermatologists recommend considering the “ugly duckling” sign: A lesion that stands out as different from the patient’s other nevi should be evaluated and possibly biopsied.61 This can be particularly helpful in a patient with a large number of clinically atypical nevi. Both of these approaches may help to identify amelanotic (nonpigmented) melanomas, which often do not meet the ABCD criteria. Some melanomas are not diagnosed until they become symptomatic, and whereas awareness of the symptoms of bleeding, itching, pain, and ulceration are worth noting, these usually connote deep vertical growth and are hallmarks of a late diagnosis, not an early one.
Biopsy
Biopsy of a suspicious skin lesion is necessary for an accurate diagnosis and for optimal staging. The correct way to perform such a biopsy is to make a full-thickness biopsy of the entire lesion, with a narrow (1 to 2 mm) margin of grossly normal skin. The depth of excision should include the full thickness of dermis and thus should extend into the subcutaneous tissue, but it does not need to include all of the subcutaneous tissue except in very thin patients or patients with very thick polypoid lesions that may go deep into the subcutis. This allows assessment of the architecture of the lesion, which is critical for differentiation of melanoma from Spitz nevus, and it permits an accurate measure of tumor thickness, which is critical for prognosis and affects the surgical treatment recommendations. Of importance, desmoplastic melanoma often arises from LMM and is difficult to diagnose both clinically and histologically. Shave biopsies of these lesions can often lead to failure to appreciate the desmoplastic melanoma in the dermis and may substantially delay diagnosis.
For some large lesions (e.g., >2 cm diameter) in cosmetically sensitive locations (e.g., face or genitalia), there may be a rationale for an incisional biopsy, but that also should be performed as a full-thickness skin biopsy. Ideally, it should include the most suspicious area of the lesion and also should include, if possible, a portion of the edge of the lesion where it transitions to normal skin to enable assessment of the junctional change. The incisional biopsy may be an elliptical incision or it may be a full-thickness 4- to 6-mm punch biopsy. Punch biopsies are problematic if too small, if they do not include full-thickness skin, if they are crushed during removal, if they are oriented inaccurately in the paraffin block, or if they are too small to include both the edge of the lesion and the most suspicious or most raised part of the lesion.
Orientation of the incision used for an excisional biopsy should be considered in the context of the prospect for the future need for a wider re-excision. On extremities, the incision and scar should be oriented longitudinally rather than transversely, although some exceptions may be considered near joints to avoid crossing a joint. When in doubt about the optimal orientation, it is very reasonable to perform the excisional biopsy as a simple circular excision, leaving the wound open for secondary or delayed primary closure.
Biopsy of subungual lesions is more challenging. The pigmentary changes seen in patients with subungual melanoma usually extend along the length of the nail, but the lesions usually arise at the proximal end of the nail bed. Access to that location often requires removal of all or a large part of the nail. One or more punch biopsies of the base of the nail bed often constitute the most realistic method for obtaining a biopsy of such lesions, and it may need to be repeated to be diagnostic. A punch biopsy tool can remove a circle of the nail, providing access to the nail bed for punch biopsy of the suspicious area.
Melanoma Subtypes: Histologic Growth Patterns
Classically, four main histologic growth patterns are described for melanomas, but two others are also worth mentioning.
Superficial Spreading Melanoma
The most common type is superficial spreading melanoma, which accounts for about 70% of primary cutaneous melanomas (see Fig. 94.3C). It is typical for the trunk and extremities, except on acral sites. It is associated with pagetoid growth of atypical melanocytes in the epidermis. Superficial spreading melanoma is commonly associated with sun exposure.
Nodular Melanoma
Nodular melanomas lack an RGP, may be nonpigmented, and commonly are diagnosed when relatively thick. Thus, these carry the worst prognosis of the various subtypes of melanoma. They account for about 20% of cutaneous melanomas. By definition, nodular melanomas are in VGP when recognized.
Acral Lentiginous Melanoma
ALMs account for <5% of melanomas.62 They are typically found on acral sites (subungual, palmar, plantar) and on mucosal surfaces (anorectal, nasopharyngeal, female genital tract). ALM occurs across all races and ethnicities. Its etiology is likely independent of UV light exposure. Because other cutaneous melanomas are uncommon in African, Asian, and Hispanic populations, ALMs on acral sites are proportionately more common in these populations than in fair-skinned whites. ALM is typically associated with a prolonged RGP before vertical growth; however, its locations make it harder to diagnose than other forms of melanoma. Subungual lesions can be detected by linear pigment streaks arising from the base of the nail, but these are not always evident. They can be confused with subungual hematomas, which can lead to diagnostic delay. When there is a question of whether a pigmented subungual lesion may be melanoma or a hematoma, the location of the pigment can be marked and then followed over a short interval (e.g., 3 weeks), during which time a hematoma should move toward the end of the nail, but a melanoma should not.
Subungual melanomas can also present with breakage of the nail or a nonpigmented thickening or drainage, and these are often confused with chronic fungal infections. Any concerning pigmented subungual lesion should be biopsied, but it is sometimes challenging and requires splitting or removing part of the nail. A punch biopsy near the nail bed matrix is often appropriate. In addition, when there is spontaneous chronic inflammation or breakage of the nail, biopsy for melanoma should be considered, even in the absence of pigmentation.
Lentigo Maligna Melanoma
LMMs typically occur in older individuals, in chronically sun-damaged skin, and commonly on the face. They tend to have shades of brown or black, whereas the red and blue colors seen in other melanomas are not typical of LMM. They may also develop areas of regression manifested by depigmentation of part of the lesion. Overall, LMMs account for about 10% to 20% of melanomas in the National Cancer Database experience,1 47% of melanomas of the head and neck, and only 2% of melanomas of other regions.62 LMMs usually have an extensive RGP that extends for many years before developing invasion. When melanoma is just in situ, this RGP portion is called lentigo maligna or Hutchinson freckle, as opposed to LMM. These are not to be confused with the benign pigmented macule, lentigo. Lentigo malignas evolve a VGP to become invasive LMMs at a rate estimated to be between 5% and 33%.63 LMMs are commonly diagnosed as thin lesions. However, more substantial vertical growth can occur, as seen in Figure 94.3A.
Lentiginous Melanoma
Early RGP melanomas sometimes are difficult to classify into the typical patterns of lentigo maligna, superficial spreading melanoma, or ALM. A report defined a distinct entity of lentiginous melanoma. Its features include diameter ≥1 cm, elongated and irregular rete ridges, confluent melanocytic nests and single cells over a broad area of the dermal/epidermal junction, focal pagetoid spread, cytologic atypia, and possible focal dermal fibrosis.64 Over time, this may represent a growing proportion of melanomas that have traditionally been grouped as superficial spreading melanoma, lentigo maligna, ALM, or unclassified melanomas.
Desmoplastic Melanoma
Desmoplastic melanoma is an uncommon form of melanoma, histologically manifest by dermal melanocytes in a dense stromal response. These lesions are usually nonpigmented and usually have lost the melanin production pathway. They usually stain negative for MART-1/MelanA, gp100, and tyrosinase, but they do stain for S100. The lack of pigmentation and the dense stromal response often interfere with clinical and histologic diagnosis. It occurs most commonly in the head and neck, but it may occur in other body sites.65 Desmoplastic melanoma may appear de novo as a nonpigmented skin papule or as a dermal/VGP component arising from a preexisting lentigo maligna or other pigmented junctional lesion. Desmoplastic melanomas may have neurotropic features and have been associated with a high rate of local recurrence.66 However, recent reports suggest that if adequate margins are taken, the risk of local recurrence is low.
The overall mortality risk for desmoplastic melanomas is comparable to that of other invasive melanomas of similar depth of invasion.67 Multiple studies support the contention that desmoplastic melanomas have a significantly lower risk of nodal metastases than other melanomas,68–72 with only 1.4% sentinel node positivity among 155 patients with pure desmoplastic melanoma, compared with 18.5% in those with mixed desmoplastic melanoma.67,68,71,72 There has been a debate about whether to abandon histologic staging of regional nodes in patients with desmoplastic melanoma.69 It may be appropriate to consider a higher threshold for performing sentinel node biopsy (SNBx) in patients with pure desmoplastic melanoma, but there is no consensus on this question.
Prognostic Factors for Primary Melanomas
The best predictor of metastatic risk is the depth of invasion, measured with an ocular micrometer, from the granular layer of the skin to the base of the primary lesion. This was originally described by Breslow73 and remains an important factor in staging and prognostic stratification. However, many other histologic and clinical features have relevance for estimating the risk of future metastasis and mortality. These include age, angiolymphatic invasion, mitotic rate, gender, and body site.
Depth of Invasion
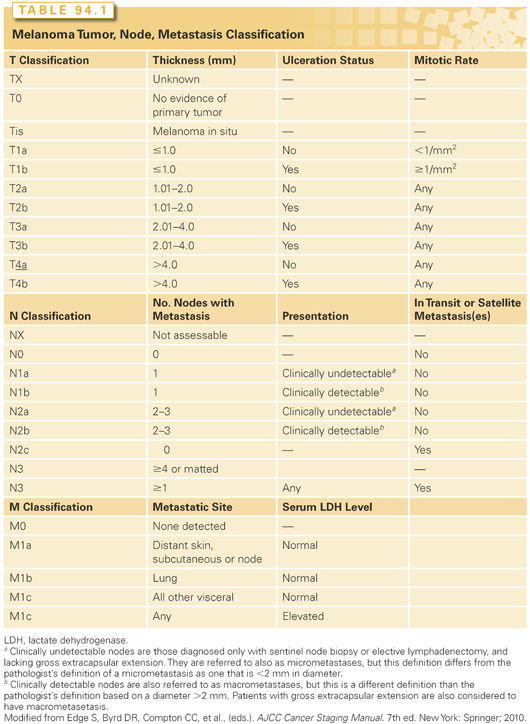
Breslow thickness is the depth of invasion measured from the granular layer of the epidermis to the base of the lesion. Melanoma cells involving adnexal structures are considered junctional and are not included in the Breslow depth. The current melanoma staging system of the American Joint Committee on Cancer (AJCC) identifies tumor (T) stage based on Breslow thickness such that T1 lesions are <1 mm thick, T2 lesions are 1 to 2 mm thick, T3 lesions are 2 to 4 mm thick, and T4 lesions are >4 mm thick.74
Clark et al.75 defined depth based on the layer of skin to which the melanoma has invaded. Clark level I melanomas are melanomas in situ, limited to the epidermis or dermal/epidermal junction. Clark level II melanomas invade into the superficial (papillary) dermis, and these are usually RGP lesions. Clark level III melanomas fill the papillary dermis. Clark level IV melanomas invade into the deep (reticular) dermis and have significant metastatic risk. Clark level V melanomas are uncommon and contain invasion into the subcutaneous fat.
It has become apparent that Clark level does not add much additional prognostic value to Breslow thickness and has been removed from the 2010 version of the AJCC staging system.74 Breslow thickness has an effect on survival, local, regional, and systemic recurrence rates, and that association is continuous, without any apparent breakpoints. Although the staging system requires categorization of thickness ranges, the continuous nature of the risk association should be kept in mind. Thickness is considered in defining the margins of excision for primary melanomas.76,77
Ulceration
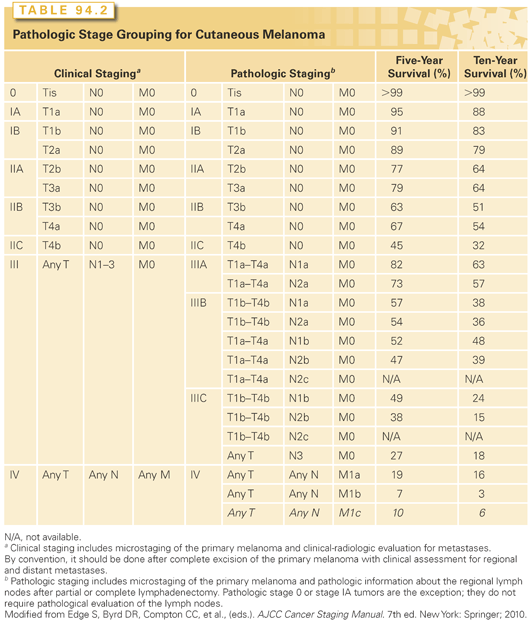
Ulceration of the primary lesion has been identified as an important negative prognostic feature76 and is incorporated in the current staging system such that T1a, T2a, T3a, and T4a melanomas are nonulcerated, and T1b, T2b, T3b, and T4b melanomas are ulcerated. In an analysis of prognostic features in a large multicenter database, the prognosis of an ulcerated lesion was comparable to that of a nonulcerated lesion one T level higher. Thus, the overall stage assignment groups ulcerated lesions with nonulcerated lesions one T level higher (e.g., T2b and T3a are both stage IIA). The staging system is summarized in Tables 94.1 and 94.2 and is described in detail elsewhere.74
Patient Gender and Skin Location of Primary Melanoma
The incidence of melanoma is higher for men than women overall, but in adolescents and young adults it is more common in women.25 Furthermore, for essentially all patient subgroups, the prognosis is better for women than men. Thus, among patients with stage III and IV melanoma, men outnumber women approximately 1.5:1. Women are more likely to have melanomas on the extremities, whereas men are more likely to have melanomas on the trunk and head and neck. The clinical outcome for patients with melanomas on extremities is better than that for patients with truncal or head-and-neck melanomas; thus, the prognostic impact of gender is difficult to distinguish from the impact of tumor location. There may still be, however, a prognostic benefit for female gender independent of tumor location.76,78 In addition, location of tumors has prognostic relevance in that head-and-neck melanomas have poorer prognosis than trunk or extremity melanomas, and melanomas on acral sites have poorer prognosis than other extremity melanomas.78,79 A particular location associated with poor prognosis is the mucosal melanoma. Anorectal, female genital, and head-and-neck melanomas of mucosal origin have a mortality risk of 68% to 89% over 5 years.1,79,80
Patient Age
The impact of age on prognosis is confusing. There is a greater risk of lymph node metastasis in young patients at the time of SNBx,81 especially for patients younger than age 35 years, but the melanoma-associated mortality risk increases with age for all thickness ranges.1,76 This paradox has not been explained. It suggests a possible age-specific curative potential for patients with micrometastatic nodal disease. Alternatively, it is worth considering that the attribution of mortality to melanoma progression is not always straightforward. Older patients have other competing causes for death that could lead to earlier mortality in the presence of metastatic disease. Nonetheless, age does appear to have independent prognostic significance for patients with melanoma.
Growth Pattern
Overall, nodular melanomas have the worst prognosis, associated with their diagnosis at a thicker stage. Lesser risk is associated with ALM, superficial spreading melanoma, and LMM, in that order, all associated with decreasing average Breslow thickness. Generally, the histologic growth pattern of melanoma has little prognostic relevance when Breslow thickness is taken into account. The VGP component appears to be the component of melanoma that determines metastatic risk, and these VGP components are similar, independent of the growth phase in the RGP component. LMMs are a possible exception, in that they appear to have a better prognosis than other histologic types, independent of thickness. Desmoplastic melanoma, superficial spreading melanoma, LMM, and ALM have comparable prognosis, for distant metastases and survival, when stratified by thickness.68,81
Mitotic Rate
It is reasonable to expect that the growth rate of melanomas is linked to the rate of tumor cell division. Accordingly, mitotic rate in the dermal component has been identified as a negative prognostic feature, especially with six or more mitoses per square millimeter.81,82 Similarly, dermal expression of Ki67, a molecular marker of proliferation, is associated with greater risk of metastasis.83 For thin melanomas, the presence of any mitotic figures has been associated with metastatic risk, whereas the absence of dermal mitoses is associated with an excellent prognosis.84 The current staging system incorporates mitotic rate of ≥1/mm2 in differentiating low-risk thin melanomas (T1a) from higher-risk thin melanomas (T1b), and data used to define the current staging system identify increasing risk with increasing mitotic rate for all thicknesses.74 Increased mitotic rate is associated with a poorer prognosis across all thickness ranges, but is not yet incorporated formally in the staging system beyond the current cutoff of 1/mm2 for thin lesions.74,85,86
Other Prognostic Factors
There is also evidence, and biologic rationale, that angiolymphatic invasion has negative prognostic significance,81 and that microscopic satellites are associated with poorer prognosis. Satellitosis is incorporated in the current staging system74 but will be considered separately because it defines the patient as stage III and thus goes beyond assessment of risk factors of the primary lesion alone.
Unresolved Issues in Melanoma Staging
The AJCC staging system is evidence-based and accounts for several important clinical and histopathologic findings. However, several clinical settings are not fully addressed by the AJCC staging system. These include the following.
Positive Deep Margin on Biopsy
When a primary melanoma is diagnosed by shave biopsy, and the tumor extends to the deep margin, it is presumed that the melanoma was deeper than the original measured biopsy depth. Sometimes, on wide local excision there is residual melanoma with a greater depth than on the original biopsy. In that setting, it is appropriate to define the T stage based on the latter depth of invasion. However, in many cases, the wide excision does not reveal any more melanoma, or may reveal tumor that is more superficial. It is generally assumed that in those cases, any residual melanoma at the deep margin may have been destroyed by inflammatory changes after the biopsy. One approach for defining T stage in that setting is to call it TX. The other is to use the T stage of the original depth, even though that is incomplete. The latter has the advantage of distinguishing thin melanomas (e.g., a clinically thin melanoma with thickness <1 mm) from a thick melanoma (e.g., a 5-mm melanoma on shave biopsy, with positive deep margin). Thus, use of TX results in substantial loss of information for patients and their clinicians.
Local Recurrence After Original Incomplete Excision
Some patients present with melanoma after excisional biopsy or destruction (e.g., cryotherapy) of a pigmented skin lesion that was believed to be benign (clinically or histologically) on initial review. When such a lesion recurs and is found to contain melanoma, re-review of the original biopsy is appropriate, if available. Staging of such recurrent melanomas, when the original lesion was not known to be melanoma, is not well addressed.
Skin or Subcutaneous Lesion Without Junctional Involvement and Without Known Primary Melanoma
This is addressed later in this chapter. Cutaneous or subcutaneous nodules that occur in the absence of junctional melanocytic change, and in the absence of any other known primary, are among the most interesting presentations of melanoma. They may be in-transit metastases from primary melanomas that spontaneously regressed (stage IIIB), primary melanomas that arose from dermal nevi or that persisted in the dermis after arising from a partially regressed primary melanoma (stage IIB), or a distant metastasis from an unknown primary melanoma (stage IV, M1a). A review of experience with these lesions at the University of Michigan suggests that they behave more like primary tumors arising in the dermis or subcutaneous tissue.76 In the current staging system, these are considered stage III.
GENERAL CONSIDERATIONS IN CLINICAL MANAGEMENT OF A NEWLY DIAGNOSED CUTANEOUS MELANOMA (STAGE I–II)
Most melanomas present as clinically localized lesions without clinical or radiologic evidence of metastatic disease. Nonetheless, some of these patients have occult metastases, and the definitive surgical management includes both therapeutic resection and pathologic staging evaluation for regional metastases. The vast majority of primary melanomas are diagnosed on histologic assessment of skin biopsy performed by a dermatologist or a primary care practitioner. The patient then presents to a surgeon or other physician for definitive treatment.
Clinical Evaluation and Radiologic Studies for Patients with Clinical Stage I–II Melanoma
In patients with clinically localized melanoma, there is a wide range of clinical practice in the appropriate radiologic staging studies to be performed. Certainly all patients with such disease should have a complete history and physical examination, with attention to symptoms that may represent metastatic melanoma, including headaches, bone pain, weight loss, gastrointestinal symptoms, and any new physical complaints. Physical examination should carefully assess the site of the primary melanoma for clinical evidence of persistent disease and should evaluate the skin of the entire region (e.g., whole extremity or quadrant of torso, or side of the face) for dermal or subcutaneous nodules that could represent satellite or in-transit metastases. Biopsy should be done for any suspicious lesions and with a very low threshold for biopsy. In addition, physical examination should include thorough evaluation of both the major regional nodal basins (e.g., epitrochlear and axillary for a forearm melanoma) and also any atypical lymph node locations, such as the triangular intermuscular space on the back for upper back primaries.
There is a great deal of uncertainty and debate about appropriate initial staging studies. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) from 2013 recommend no staging radiographs or blood work for melanoma in situ, and recommend imaging for low-risk thin melanomas (stage IA) “only to evaluate specific signs or symptoms.” For clinical stage I–II, no other imaging is recommended. For stage III melanoma, consideration of imaging is recommended, to include chest radiograph (CXR), computed tomography (CT) scans, or positron emission tomography (PET)/CT scans, with consideration of magnetic resonance imaging (MRI) of the brain, and other imaging is suggested only as clinically indicated. More complete staging studies are suggested for stage III melanoma.87
CXR for asymptomatic patients with a new diagnosis of clinically localized melanoma yielded suspicious findings in 15% of patients, of whom only 0.1% had a true unsuspected lung metastasis.88 In a similar study, the yield of true positive CXR was 0% of 248 patients.89 In patients with stage IIB melanoma, initial staging CT scans identified occult metastasis that changed management in 0.7% of patients.90 Even in patients with positive SNBx, staging PET scan identified no melanoma metastases in 30 patients, even though there were lymph node metastases in 16% of cases.91 In patients with clinical T1b–T3b melanomas, true positive rates for all imaging studies was 0.3%, and false-positive rates were 50% to 100% for CXR, 88% for CT and PET/CT scans.92 Thus, there is a large body of data that argues that CXR, CT, and PET/CT are all of little or no value in initial staging of melanoma stage 0–IIIA.
PET with fluorodeoxyglucose (FDG) has a role in staging patients with advanced melanoma,93 but its role in earlier-stage disease is less clear both because it is expensive and because it is associated with substantial radiation exposure. In one study, patients with clinically localized melanomas >1 mm thick, with local recurrence, or solitary in-transit metastases, FDG-PET scanning was performed prior to sentinel node biopsy. Sensitivity for detection of sentinel nodes was only 21%, although specificity was high (97%). In addition, 21% of patients had PET evidence of metastases, but none was confirmed by conventional imaging at that time, and the sensitivity for predicting sites of future disease recurrence was only 11%. Overall sensitivity for detecting occult stage IV disease was only 4%, and this is not recommended for initial staging.94 These findings are similar to other experiences with PET imaging for intermediate-thickness melanomas.95,96
Also, some clinicians send blood for a complete blood count, for serum chemistries, including liver function tests, and for a lactate dehydrogenase (LDH) level, especially as they may be useful prior to surgery under general anesthesia. These also are of low clinical yield in terms of the melanoma but may detect unappreciated concurrent illness that may affect therapeutic decisions, including preoperative assessment. Specifically, if there is microcytic anemia, it should be worked up, with the differential diagnosis to include gastrointestinal metastasis of melanoma. Elevated LDH should prompt a more extensive staging workup, and elevated liver function tests should prompt a hepatobiliary ultrasound or CT scan unless there is another known explanation.
Wide Local Excision for Clinical Stage I–II Melanoma: General Considerations
Wide excision of the primary melanoma is performed to provide local control. Multiple randomized, prospective clinical trials support current recommendations for the extent of the margins of resection. The wide excision also provides an opportunity to evaluate the tissue adjacent to the primary lesion for microscopic satellites, which, if present, have clinical and prognostic significance.
There has been considerable debate about the appropriate margins of excision for primary melanomas, and it is helpful to understand the evolution of thought and data about this topic. In the early 1900s, melanoma was a rare disease, and when it was diagnosed, it was often locally advanced. Surgical resection was often associated with recurrence disease, and there were no guidelines for appropriate and successful surgical management of the primary lesion. In 1907, Handley reported a study that involved histologic examination of tissue sections taken at varied distances from the primary melanoma in a human tissue specimen that he obtained from a patient with a large primary melanoma. In that study, he found microscopic evidence of melanoma cells as far as 5 cm from the primary tumor. He recommended wide re-excision of melanomas with a measured margin of 5 cm from the primary lesion. This recommendation became standard management for melanoma for many decades, with patients typically undergoing radical resections requiring skin grafts ≥10 cm in diameter.
As melanoma became a more frequent diagnosis, there was greater awareness of it, and lesions were often diagnosed at an earlier (thinner) stage. In addition, these large re-excisions usually contained no detectable melanoma cells separate from the primary lesion. These observations, and concern for the morbidity of large resections and skin grafts, led to a questioning of the need for 5-cm margins of resection. It is ironic that the origin of this aggressive resection practice was based on data from a single patient in a single study; however, limiting the margins of excision has required multiple large, randomized, prospective trials. These trials are summarized in Table 94.3 and are detailed in the follow sections.
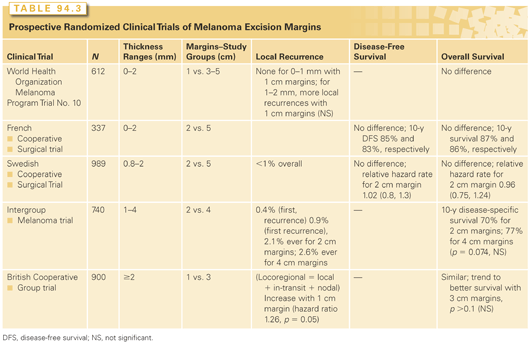
CLINICAL TRIALS TO DEFINE MARGINS OF EXCISION FOR PRIMARY CUTANEOUS MELANOMAS
The World Health Organization (WHO) Melanoma Program Trial No. 10 randomized 612 melanoma patients with melanomas ≤2 mm in thickness to excision margins of 1 cm versus 3 to 5 cm.97,98 Patients were stratified into two subgroups: Breslow depth <1 mm versus 1 to 2 mm. There were no differences in survival rates or in rates of distant recurrences with 1-cm margins versus 3- to 5-cm margins with follow-up beyond 15 years.99 There were more local recurrences for the group with 1-cm margin (eight versus three patients), but this was not a significant difference. There were no local recurrences for melanomas <1 mm thick treated with 1-cm margins. The lack of local recurrences with thin melanomas (<1 mm) after 1-cm margins of excision support this as a standard excision margin for T1 melanomas. The numerically slightly higher (but statistically insignificant) local recurrence risk with thinner margins for T2 melanomas has left questions about the appropriate margin for thicker lesions.
French and Swedish Cooperative Surgical Trials
The French Cooperative Group randomized 337 patients with melanomas up to 2 mm in thickness to 2- or 5-cm margins.100 Ten-year disease-free survival rates were 85% and 83%, respectively, and ten-year overall survival (OS) rates were 87% and 86%, respectively.100 The Swedish Melanoma Study Group randomized 989 patients with primary melanoma 0.8 to 2 mm thick on the trunk or extremities to 2- or 5-cm margins. Local recurrences were observed in only eight patients overall (<1%). In a multivariate Cox analysis, estimated hazard rates for OS and recurrence-free survival for those with 2-cm margin were 0.96 (95% CI, 0.75 to 1.24) and 1.02 (95% CI, 0.8 to 1.3), respectively, compared with the 5-cm margins.101 Both of these studies support 2-cm margins as adequate for melanomas up to 2 mm thick and find no added benefit to 5-cm margins.
Intergroup Melanoma Trial
The Intergroup Melanoma Surgical Trial addressed the question of surgical margins in 740 patients with intermediate-thickness melanomas (1.0 to 4.0 mm thick) randomized to either 2- or 4-cm margins.102 Patients were stratified by tumor thickness (1 to 2 mm, 2 to 3 mm, and 3 to 4 mm), anatomic site (trunk, head and neck, and extremity), and ulceration (present or absent). Patients with melanomas on the head and neck or distal extremity were not randomized for margin of excision because 4-cm margins are not readily performed in such locations. Thus, 468 patients (group A) were actually randomized for margin of excision. All patients were also randomly assigned to undergo either an elective lymph node dissection (ELND) or observation after wide local excision, and this component of that study is discussed separately.102
Among the 468 patients in group A (randomly assigned to excision with 2- versus 4-cm margins), only 3 (0.6%) experienced a local recurrence as the first site of failure, and 11 (2.3%) had local recurrence overall.102 Among the 272 patients in group B (nonrandomly assigned to excision with a 2-cm margin), a higher rate of local recurrence was observed, with 3.7% having a local recurrence as a first recurrence and 6.2% overall experiencing a local recurrence during the course of their disease.102 Among these 468 patients in group A, the incidences of local recurrence as first relapse were 0.4% versus 0.9% for 2- and 4-cm margins, respectively, and the incidences of local recurrence at any time were 2.1% versus 2.6%, respectively. In addition, the time to local recurrence and the median survival after local recurrence were unaffected by the extent of the margin. Ten-year disease-specific survival rates for the two groups were 70% and 77% for 2- and 4-cm margins, respectively (p = 0.074, not significant). Thus, this study supports a 2-cm margin as adequate for melanomas 1 to 4 cm thick, and this was associated with rates of local recurrence (as first recurrence) well <1%. Multivariate analysis of data from this study further supported the lack of benefit of wider margin of excision for local control and identified ulceration of the tumor and head-and-neck location only as significant negative prognostic features.
British Cooperative Group Trial
The British randomized trial compared 1- versus 3-cm margins of excision in patients who had cutaneous melanomas ≥2 mm thick (T3, T4).103 Nine hundred patients with T3 and T4 melanomas were accrued, of whom 25% had T4 melanomas. It is the only randomized trial evaluating margins of excision that included patients with T4 melanomas. Patients with melanoma on head and neck, hands, or feet were excluded. No patients had any surgical procedure to stage the regional nodal basins (sentinel node biopsy or ELND) or systemic adjuvant therapy. The trial was stratified according to tumor thickness (2 to 4 mm versus >4 mm). There were few local recurrences; local recurrences and in-transit metastases were not statistically more frequent in the 1-cm margin group. Locoregional recurrences were defined broadly to include local, in-transit, or regional nodal recurrences. Using that definition, a 1-cm margin of excision was associated with a significantly increased risk of locoregional recurrence (hazard ratio [HR], 1.26; p = 0.05). Overall survival was comparable for the two groups (p = 0.6); there was a nonsignificant trend toward higher death rate in the group with 1-cm margins (128 versus 105 deaths; HR 1.24, p = 0.1). This study has been controversial, and its relevance to current practice is questioned because of the lack of surgical staging of the regional nodes, but it does challenge the safety of 1-cm margins for melanomas >2 mm thick.103 These results support excision >1 cm for thicker melanomas. The data from the Melanoma Intergroup study support 2-cm margins for melanomas 2 to 4 mm thick. No data have formally compared 2-cm margins with 3-cm margins for T4 melanomas.
SURGICAL STAGING OF REGIONAL NODES
Thin and RGP melanomas are commonly cured by excision alone; however, thicker melanomas may have metastatic potential. Initial management includes an assessment for metastases and consideration of treatment options that may be beneficial in providing regional control and systemic control. Melanoma may metastasize by lymphatic or hematogenous routes. Usually, lymphatic dissemination presents earlier than hematogenous dissemination. Thus, emphasis is placed on staging the regional nodes in patients with melanoma. The finding of lymphatic metastases is associated with a higher risk of systemic disease. Another potential benefit of staging the regional nodes is to select patients for curative resection. There are substantial data on this issue that bear on the current recommendations for surgical staging of nodes, and these are summarized here.
Lymphatic anatomy is variable and is poorly understood in comparison to venous and arterial anatomy. Classic work by Sappey defined aspects of lymphatic drainage patterns from skin and defined the skin regions that typically have lymphatic drainage to major nodal basins. More recently, lymphoscintigraphy has permitted mapping the actual lymphatic drainage patterns from the skin at the site of the primary melanoma. This sometimes identifies lymphatic drainage that differs from Sappey’s predictions.
In the past, the standard recommendation was to perform ELNDs for melanomas of intermediate thickness (1 to 4 mm). Despite some retrospective data supporting this approach,104 subsequent retrospective and prospective studies have failed to show a significant survival advantage to routine ELND.105–107 In the early 1990s, a new procedure was developed and popularized for surgical staging of node-negative primary melanomas, which is called intraoperative lymphatic mapping and sentinel lymph node biopsy. This approach has become routine practice for melanoma management.
The concept and method for SNBx was originally developed by Cabanas108 for management of penile carcinomas, but it was not pursued extensively. The initial experience with lymphatic mapping and SNBx for melanoma was the work of Morton et al.109 at the University of California Los Angeles and the John Wayne Cancer Institute. They injected a vital blue dye (isosulfan blue) intradermally and found that this stained the draining lymphatics and stained, in turn, the first node(s) into which these lymphatics empty. This was validated in human clinical experience and it was rapidly adopted as an effective way to identify the first lymph node(s) to which the melanoma drains.110 The sentinel node(s) serve as sentinels for the remainder of the node basin. Lymphatic mapping permits identification of the specific nodes that drain the relevant area of skin, and so these nodes (typically one or two nodes) can be excised for detailed histopathologic assessment while sparing the remaining nodes in that basin, which are critical for drainage of other skin areas, thus minimizing morbidity, in particular lymphedema.
Lymphoscintigraphy has been coupled with the blue dye injection to support identification of the sentinel node(s), using handheld probes for detection of γ radiation emitted by technetium-99 (99Tc), the radionuclide commonly used in lymphoscintigraphy. Most surgical oncologists performing SNBx use a combination of radionuclide injection several hours preoperatively (in the nuclear medicine suite, up to 1 mCi of 99Tc) and intraoperative intradermal injection of isosulfan blue dye (up to 1 ml) a few minutes prior to the incision. The injection of radiocolloid is shown in Figure 94.6. The sentinel node(s) should be both blue and radioactive (“hot”). However, sometimes the blue dye may fail to enter the node in the short interval before the dissection. Alternatively, if the dissection takes longer than anticipated, the blue dye may transit through the node by the time the node is identified. In addition, technical issues may result in the blue dye and radiocolloid being injected in slightly different areas, such that they identify different nodes. The gamma probe is used to guide the dissection to the sentinel node(s), as suggested in Figure 94.7.
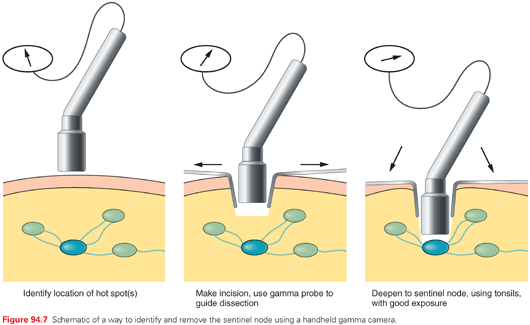
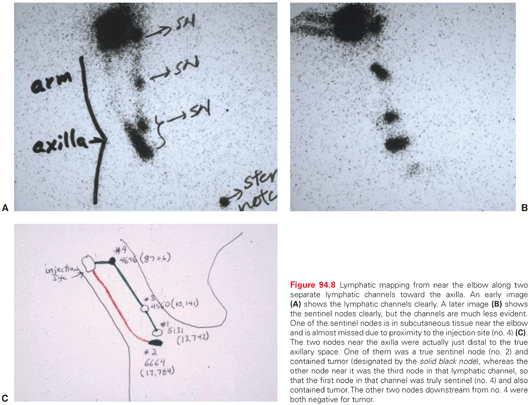
Lymphatic mapping and SNBx using both blue dye and radiocolloid increases sentinel lymph node identification rates to 99% compared with 87% with blue dye (p <0.0001).111 However, radiocolloid alone has not been formally compared with radiocolloid plus blue dye. There is substantial multicenter and single-center experience with use of radiocolloid alone, which is associated with successful identification of the sentinel node(s) in >99% of patients and with a mean of approximately two sentinel nodes per patient.112 The effectiveness of blue dye alone is limited because some patients have drainage to lymph node basins that may not be predicted (e.g., drainage from the right upper back to the left axilla) or drainage to atypical nodal basins (e.g., the triangular intermuscular space on the back, epitrochlear or popliteal nodes, or subcutaneous “in-transit” nodes that are outside a traditional nodal basin).113,114 Examples of unusual lymph node locations mapped by lymphoscintigraphy are shown in Figure 94.8. Thus, in the large majority of clinical settings, it is most appropriate to perform radiocolloid lymphoscintigraphy in lymphatic mapping for SNBx of melanoma.
In experienced hands, lymphatic mapping should identify a sentinel node in 98% to 100% of cases, and it should be feasible to perform the SNBx with minimal morbidity, on an outpatient basis, and in many cases under local anesthesia with sedation. The early reports of SNBx stress a long learning curve, but as the technology of gamma probes has improved, the technique is less operator dependent. In addition, lymphatic mapping has now been performed long enough that surgical residents trained since the mid-1990s typically have had experience with it for melanoma and for breast cancer. The standard evaluation of a sentinel node includes evaluation of multiple sections of the node, often combined with immunohistochemical staining for melanoma markers (e.g., S100, HMB45, tyrosinase, and/or MART-1/MelanA).
Typical results of SNBx reveal that the rate of positive nodes increases with increasing tumor thickness, as would be expected, from <5% for the thin melanomas that undergo SNBx (e.g., T1b lesions) to approximately 40% for thick melanomas. Current experience with SNBx in most series supports the prognostic value of SNBx in thick melanomas (>4 mm)115 as well as in thinner lesions. When ELND was performed, it was typically recommended only for melanomas 1.5 to 4 mm thick. However, in the Duke experience, the relative risk of distant versus regional metastases is not dramatically higher for thick melanomas, and this supports a clinical approach that includes the potential for curative resection of regional metastases in these cases.105 In addition, the low morbidity of SNBx supports a threshold for SNBx in thinner melanomas than the 1.5-mm criterion that was used for performance of ELND.
The overall rate of positive SNBx in most series (typically for melanomas >1 mm) is in the range of 15% to 25%. The percentage of patients with false-negative SNBx in experienced hands and with use of radiocolloid and the handheld gamma probe, with or without blue dye, is typically in the range of 1.9% to 4%.111 The most rigorous definition of false-negative rate is false negative/(false negative + true positive), and 3% false negative in the setting of 20% true positive represents 13% false-negative rate. False-negative rates have been estimated by seeking nodes containing metastases in the remaining nodal basin after a negative SNBx. In other settings, it is done by defining patients who return with clinically evident nodal metastases after a prior negative SNBx in the same node basin. These may or may not be equivalent. Nonetheless, there is a small percentage of patients who have negative SNBx who later return with nodal metastases in the same nodal basin. Although the procedure is very accurate and does identify the large majority of nodal metastases, it is prudent to follow patients for nodal recurrence even after a negative SNBx.
Lymphatic mapping and SNBx has been applied generally for all cutaneous sites and may also be useful for melanomas of mucous membranes.116 A challenging area for SNBx is the head and neck. In particular, melanomas of the scalp and of the face may drain to parotid nodes or periparotid nodes, for which SNBx is more complex, more technically challenging, and associated with greater potential morbidity. In addition, false-negative SNBx are more common than in trunk and extremity melanomas, occurring in approximately 10% of patients, for a true false-negative rate that may approach 30%. However, in many cases, it can be performed reliably and still has a place in management.
More recent technology that offers promise for improving sentinel node localization are the development of mobile gamma cameras that can replace the single gamma detector of the gamma probe with an array of hundreds of detectors that permit real-time imaging that rivals that of the fixed gamma camera. This approach has the potential to improve identification of nodes in atypical locations and for ensuring adequate clearance of the sentinel nodes.117 Also promising is single photon-emission computed tomographic/CT imaging, which can provide very discrete localization of sentinel nodes, which may be helpful in selected challenging locations. Despite the high accuracy of SNBx for nodal staging, the false-negative rate may be as high as 10% to 20%,118 and these new technologies offer a possibility to reduce that false-negative rate.
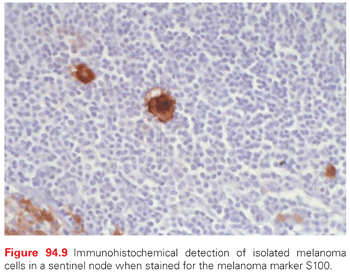
In performing SNBx, melanoma metastases are sometimes clinically evident in the operating room as small pigmented spots just under the capsule of the node. When these are present, the hottest part of the node is usually precisely at that location (unpublished clinical observations). This may be particularly relevant for some large nodes, where the pathologist can be guided to the portion most at risk of metastasis for detailed histologic assessment (Fig. 94.9). Morton et al.119 have formalized a technique that may identify the part of the node that is most likely to contain metastases, based on injecting carbon black dye and isosulfan blue dye. This has not yet become standard, but this or other refinements may further increase the accuracy of staging by this procedure.
The Multicenter Sentinel Lymphadenectomy Trial 1 was initially reported in 2006, and updated in 2014, as a randomized, prospective trial of 1,269 patients with melanomas 1 to 4 mm thick who were randomized to SNBx or observation in addition to wide local excision of the primary lesion.110,120,121 The finding was that there was no difference in 5-year disease-specific survival (87.1% versus 86.6%).120 Patients who developed clinically positive nodes during follow-up after initial observation of the node basins had worse survival than those with positive nodes found at the time of SNBx; however, this posthoc analysis carries inherent weaknesses.120 In this trial, patients were randomized 3:2 to wide excision plus SNBx, or wide excision only, respectively. In the group receiving SNBx, 225 of 814 (27.6%) patients underwent early completion lymph node dissection (CLND), compared to 132 of 533 (24.7%) in the control group having delayed CLND. Lymphedema was significantly greater (20.4% versus 12.4%, p = 0.04) for those in the observation group who underwent delayed CLND, and hospitalization was longer for delayed CLND.122 The follow-up results in 2014 supported the conclusion that biopsy-based staging of intermediate-thickness or thick primary melanomas provides important prognostic information and identifies patients with nodal metastases who may benefit from immediate CLND. In this series, SNBx-based management prolonged disease-free survival for all patients and prolonged distant disease-free survival and melanoma-specific survival for patients with nodal metastases from intermediate-thickness melanomas.121
One important consideration should be kept in mind, which is often overlooked in considering the potential value of SNBx and subsequent CLND. That consideration is the value to patients of regional control of their tumor, even in the absence of survival benefit. A study evaluating patients’ perception of their own utilities for health states suggested that the development of recurrent disease markedly decreases patient perception of their health state, even if it does not impact survival.123 This study thus suggests that regional tumor control may have value to patients, even in the absence of a survival benefit.
The rationale for performing SNBx for melanoma includes the following: (1) A negative SNBx is a good prognostic indicator that may provide comfort to low-risk patients. (2) A positive SNBx for patients with T1–T3a clinically N0 melanomas (clinical stage I–IIA) renders them candidates for adjuvant high-dose interferon (HDI) therapy, which offers some clinical benefit. (3) Patients with T4 melanomas or with microscopic satellites (N2c, stage IIIB) are further upstaged by the finding of a positive sentinel node, which helps these patients in risk assessment and may make them candidates for selected clinical trials. (4) Many clinical trials require surgical staging of regional nodes, and, thus, sentinel node mapping makes patients candidates for trials that may prove to be of benefit. (5) Identification of melanoma in a sentinel node permits selection of patients for CLND to increase the chance of regional tumor control. (6) Excision of the sentinel node may be curative if there is no tumor beyond the node, even if CLND is not feasible. This hypothesis is being explored explicitly in the Multicenter Selective Lymphadenectomy Trial 2 (MSLT2).
Selection of Patients for Sentinel Node Biopsy
SNBx is generally recommended for patients with melanomas at least 1 mm thick. For thinner melanomas, there is debate about the appropriate criteria for performing SNBx.81 A common practice is to offer SNBx for thin melanomas with adverse prognostic features, including ulceration. The 2010 staging system also identifies a mitotic rate of ≥1 as an adverse prognostic feature, and this is associated with higher risk of sentinel node metastasis. Earlier data also support the relevance of mitotic rate as a prognostic factor in primary melanomas82 or Clark level IV.78 There is debate about performing SNBx for thin melanomas that are in VGP, that have dermal mitoses, or that occur in young patients.81,84 Also, there is rationale for offering SNBx for melanomas <1 mm thick that have a positive deep margin on biopsy and thus are not fully evaluable for depth.
Pure desmoplastic melanomas have a similar overall metastatic and mortality risk as other melanomas, but their risk of regional nodal metastases appears to be lower than that of other melanomas.67,124 Thus, it may be reasonable to limit SNBx for pure desmoplastic melanomas. However, there is limited experience with managing regional nodes in desmoplastic melanoma, and some desmoplastic melanomas can metastasize to regional nodes.65
Sentinel Node Biopsy Subsequent to a Prior Wide Local Excision
SNBx should be performed at the same procedure as wide local excision. However, there are some circumstances in which wide local excision may be performed without SNBx, and there is then a question of whether SNBx can be performed reliably after a prior wide local excision. Such circumstances include a thin melanoma on original biopsy, found to be deeper on re-excision or on second-opinion pathology review. A multicenter experience with 76 patients having SNBx performed after a prior wide local excision revealed a 99% success rate in SNBx, a mean yield of two sentinel nodes per patient, with a 15% overall sentinel node–positive rate, a 4% rate of melanoma recurrence in a negative mapped basin, and only a 1% rate of isolated first recurrence in a node. These and other data support performing SNBx after prior wide local excision, although performing it concurrently with the original wide local excision is preferred.125
Clinically Localized Melanoma
Melanoma In Situ (Clinical TISN0M0, Stage 0)
For melanomas confined to the epidermis and epidermal/dermal junction that are diagnosed as melanoma in situ, this is a lesion that is curable in the vast majority of cases by wide excision alone. On initial evaluation, the regional nodes should be examined, as should the skin and subcutaneous tissue between the primary site and these regional node basins. Melanoma in situ by definition is not invasive or metastatic; however, metastatic melanoma to regional nodes has been observed occasionally from melanoma in situ with histologic evidence of regression.126 Thus, it is prudent to examine the nodes clinically. However, in the absence of clinical evidence of metastasis, there is no need to perform radiologic staging studies. Definitive management involves re-excision with a margin of 5 mm. The standard recommendation is to perform a full-thickness re-excision including underlying subcutaneous tissue, although there are no formal data that a full-thickness skin excision is less adequate for melanoma in situ. However, variation in thickness within the original biopsy specimen may lead to occult invasion that is not observed on the evaluated sections. Thus, it is prudent to perform a full-thickness excision of skin and subcutaneous tissue to the underlying deep fascia. A 5-mm margin is the standard recommendation, but melanoma in situ can extend beyond its visible extent. Thus, if cosmetically acceptable, it is reasonable to obtain a margin of as much as 1 cm, especially if the original biopsy was incomplete. If the margins are positive or close, re-excision to a widely clear margin is recommended. SNBx is not indicated. No adjuvant therapy is needed if the margins are widely clear.
Clinical Follow-Up After Surgical Treatment
Melanomas in situ are curable in the vast majority of cases with surgery alone. However, they rarely may be associated with metastasis, probably attributable either to an invasive component that was not detected because of sampling error, or to an associated regressed invasive component.126
Thus, in accord with the NCCN Guidelines®, it is appropriate to follow these patients for local recurrence, in-transit metastasis, or regional node metastasis on an annual basis. The risk of recurrence is not high enough to require specialty follow-up, but a focused physical examination of the patient by the primary care physician is appropriate. More important, patients with melanoma in situ are at increased risk of subsequent primary melanomas, and so close dermatologic follow-up with full-body skin examinations is recommended.
Thin Primary Melanoma (Clinical T1A)
The classic definition of a thin melanoma was based on the original report of Breslow73 of the association between depth of invasion (Breslow thickness) and subsequent risk of metastasis and death. In that report, patients with melanomas <0.76 mm thick had no subsequent metastasis. Thus, the definition of a thin melanoma had been a melanoma <0.76 mm thick. However, subsequent studies have shown a continuous risk association with increasing thickness, without an absolute “cutoff” at 0.76 mm,73 and melanomas <0.76 mm in thickness do have approximately a 5% risk of subsequent metastasis.127 Additional studies have defined additional histopathologic features that affect the prognosis of thin melanomas. The current AJCC staging system addresses several prognostic features of thin melanomas such that T1a melanomas are those <1 mm thick, with less than one mitosis per square millimeter, and without ulceration. In the absence of any clinical evidence of metastasis, these are clinical stage IA melanomas and have a 5-year survival rate of 94%.74,78
In most centers, the surgical management of patients with T1a melanomas includes wide excision with a 1-cm margin (including skin and all underlying subcutaneous tissue, to the deep muscle fascia). The margin should be measured from the visible edge of the pigmented lesion or from the biopsy scar, whichever is larger. Excisions of this size can almost always be closed primarily, with exceptions being on the face, palms, and feet, where skin grafts or rotation flaps may be needed.
Surgical Methods in Wide Local Excision (Applies for All Primary Melanoma Thicknesses)
For melanomas of the trunk and proximal extremities, wide local excisions should involve measuring the appropriate margin (usually 1 to 2 cm) around the entire scar from the biopsy, or from the visible edge of residual melanoma, and extending the incision to make an ellipse that is approximately three times as long as it is wide. Ideally, the direction of the scar should be longitudinal on the extremities, occasionally with some modification at joints, and should be along skin lines on the trunk and neck. On the upper back, it is usually best for the scar to run transversely, to minimize tension on it. When the initial biopsy scar is not in the direction that is desired for the final excision, an effective approach is first to mark out the oval shape that is required for the appropriate margins, then rather than extending that to an ellipse that is in the same direction, the ends of that oval can be extended in the desired direction, resulting in a sigmoid-shaped oval, which has two advantages: The closure results in a scar that is more in the desired direction, and the sigmoid shape allows the tension to be distributed in two directions. The excision should include all skin and subcutaneous tissue to the deep fascia, but not including the fascia. When a major cutaneous nerve runs along the deep fascia to innervate distal cutaneous structures, it is appropriate to preserve that nerve. Wide excisions can almost always be performed under local anesthesia, with or without intravenous sedation, in the patient who is thus motivated.
Clinical Follow-Up for Thin Melanomas (Stage IA)
There are no definitive data showing a survival advantage for close follow-up after surgical management of primary or metastatic melanoma; however, there is an expectation from patients for follow-up, and there are treatable recurrences and metastases that can be identified best by physician follow-up. The National Comprehensive Cancer Network® (NCCN®) has issued useful guidelines for treatment and follow-up of melanoma.128 The risk of metastasis for thin melanomas is in the 5% to 10% range, and less for RGP lesions. In the uncommon case of recurrent thin melanomas, the recurrences usually occur late, often beyond 5 years from diagnosis; the annual risk of recurrence is fairly constant over a long time,47 so annual follow-up for many years is recommended rather than frequent follow-up in the first few years. Follow-up suggestions are listed in Table 94.7.
Clinical T2A, T2B Melanomas
Melanomas 1 to 2 mm thick, with or without ulceration, should be managed with an initial history and physical examination to elucidate signs or symptoms that could suggest metastatic disease. In the absence of such findings, there is very low yield of additional staging studies, and they are not recommended. In those patients without evidence of metastasis, definitive management includes wide excision with a 1- to 2-cm margin and SNBx. There are definitive data from the Melanoma Intergroup trial that a 2-cm margin is adequate for these patients,107 and even a 1-cm margin was associated with the same survival as a 3- to 5-cm margin in long follow-up of the WHO Trial 10 (see Table 94.3).99 However, there has been a slight increase in local recurrence in patients with 1- to 2-mm lesions who had 1-cm margins (versus 3- to 5-cm margins). This is not statistically significant in the patients studied, but it may signal a slight increase in local recurrence risk. When it is feasible to take a 2-cm margin without a skin graft (trunk and proximal extremities in most cases), this is recommended to minimize the chance of local recurrence. However, when the lesion is located on the face or distal extremities, where such a margin may be difficult to achieve without a skin graft, a 1- to 1.5-cm margin is acceptable. If a skin graft will be necessary even to close a 1-cm margin (rare), it is recommended that a 2-cm margin be taken because the morbidity and cost of the skin graft will already be needed. In addition, for lesions that are barely above 1 mm in depth (e.g., 1.03 mm), it certainly is reasonable to use a 1-cm margin.
SNBx is routinely recommended for patients with melanomas 1 to 2 mm thick.129 If the SNBx is positive, then subsequent management should follow recommendations given later for stage IIIA melanoma (T2a with positive SNBx involving one to three nodes) or stage IIIB melanoma (T2b with positive SNBx involving one to three nodes). However, if the SNBx is negative, then the patient is considered to have been pathologically staged as T2aN0M0 (stage IB) or T2bN0M0 (stage IIA), and no additional surgical management is required and no adjuvant systemic therapy is indicated, other than clinical trials.
Clinical T3A Melanomas (Clinical Stage IIA)
Melanomas 2 to 4 mm thick, without ulceration, represent T3a lesions, and in the absence of metastases, these are clinical stage IIA lesions. They should be managed clinically with a history and physical examination as detailed previously and may be considered for a staging studies and serum LDH level. Definitive management includes wide excision with a 2-cm margin and SNBx for histologic staging of the regional nodes. If the SNBx is negative, then no additional surgical or systemic therapy is indicated other than possible clinical trials. If the SNBx is positive, then management for stage IIIA melanoma should be followed.
Clinical T3B Melanomas (Clinical Stage IIB)
Melanomas 2 to 4 mm thick with ulceration represent T3b lesions and thus are clinical stage IIB melanomas. These are high-risk localized melanomas. Initial management should include a careful history and physical examination. Staging studies are generally of low yield, but in selected high risk cases may be considered, and if there are symptoms suspicious for metastatic disease, there is value in performing indicated imaging studies.130 Given the higher risk of synchronous metastases that may be detected at diagnosis, systemic staging with CT scans of the chest, abdomen, and pelvis (or PET/CT scan) plus MRI scan of the brain may be indicated if there are symptoms or signs suggestive of systemic metastasis.
In the absence of clinical evidence of metastasis, definitive management is wide excision with a 2-cm margin and an SNBx. If the nodes are negative, the summary stage is IIB (T3bN0M0). For these patients, no additional surgical therapy is needed. However, HDI and pegylated-interferon therapies have been approved for use as postsurgical adjuvant therapy for patients with resected stage IIB-III melanoma. It is worth noting that the randomized clinical trials of adjuvant interferon were performed before the recent revision of the AJCC staging system, when ulceration was not incorporated in the staging system. Thus, the patients with stage IIB in whom interferon was tested did not include the current patients with stage T3bN0. Nonetheless, it is available for such patients, whose risk is comparable to that of patients with nonulcerated thick melanomas (T4aN0).
Thick Melanomas (T4A, T4B, Greater than 4 mm Thick)
Thick melanomas have been commonly associated with a risk of metastasis and mortality in the range of 50% over 5 to 10 years. Ulceration increases this risk: T4a melanomas are clinical stage IIB, and T4b melanomas are clinical stage IIC. Initial workup should include a history and physical examination, and serum LDH plus more aggressive radiologic imaging as indicated by signs and symptoms. For these high-risk patients, consideration should be given to more complete staging with CT scans of the chest, abdomen, and pelvis plus MRI of the head. Definitive management includes wide excision with at least a 2-cm margin plus SNBx. There are no definitive prospective, randomized data regarding margins for melanomas thicker than 4 mm, but margins of at least 2 cm are recommended. The general experience is that 2-cm margins provide adequate local control for these lesions, suggesting that the strong data supporting the adequacy of 2-cm margins in 1- to 4-mm melanomas may be extrapolated to thicker lesions.131
As SNBx has been employed routinely since the early 1990s, most studies show that sentinel node status has independent prognostic value for patients with thick melanomas.131 Because these patients have a high risk of sentinel node positivity (approximately 35% to 40%), there is a high chance of regional nodal recurrence, and SNBx, followed by CLND, offers the prospect of increasing the chance of regional control. In patients with negative sentinel nodes, adjuvant interferon should be considered because it is approved by the FDA for these patients. This should be discussed in detail with patients.
SPECIAL CONSIDERATIONS IN MANAGEMENT OF PRIMARY MELANOMAS
Primary Melanomas of the Head and Neck
For melanomas on the head and neck, there are important anatomic constraints, and there are times when the optimal margins are not feasible (e.g., a 2-cm margin for a lesion 1 cm below the eye), but to the extent possible, the optimal margins should be obtained and closed with an advancement flap, skin graft, or limited rotation flap. In the unusual circumstance of a large-diameter lentigo maligna on the face that is not amenable to surgical resection because of cosmetic results or comorbid patient conditions, it may be treated with superficial or Grenz X-rays with local control rates reported above 90%.132 Anecdotal reports of off-label topical treatment with imiquimod ointment have also resulted in effective local control of superficial melanomas.133,134 This is being used increasingly, with good results in reported experience, but recurrence may occur.135 Initial experience suggests that imiquimod is not effective at eradicating dysplastic nevi.136 Desmoplastic melanomas commonly occur in the head and neck region and may have reported local recurrence rates up to 40% to 60% after resection.137 Other series vary substantially in local recurrence rates of desmoplastic melanomas. One reports local recurrences as first recurrences in 14% of patients, which exceeds that of other histologic types,69 and another reports no difference in local recurrence rates compared to other melanomas, although the presence of neurotropism was associated with higher risks of local recurrence.70 An explanation for the high local recurrence rates in some series of desmoplastic melanoma may include inadequate margins of excision because of anatomic constraints in the head and neck. In addition, because desmoplastic melanomas are usually amelanotic, the surgical margins may be underestimated, and the histologic appearance of desmoplastic melanoma can interfere with accurate detection of microscopically positive margins, especially in fibrotic skin. Thus, in patients with desmoplastic melanoma, every effort should be made to obtain adequate margins.137 If that is not possible, postoperative adjuvant radiation should be considered with 2- to 3-cm margins around the resected lesion because this may reduce subsequent local recurrences.
Neurotropic melanomas of the head and neck have a propensity to recur at the skull base by tracking along cranial nerves, and postoperative adjuvant radiation including the resection bed and the cranial nerve pathway should be considered for this variant.
Primary Melanomas of the Mucous Membranes
Mucosal melanomas of the head and neck, anorectal region, and female genital tract are usually diagnosed when they are thick. They are associated with higher risks of distant metastases and death compared to cutaneous melanoma. They are also associated with higher risks of local recurrence and regional nodal metastases. Staging of these lesions is not addressed completely in the AJCC staging system for cutaneous melanomas, but there are general similarities that can be applied to mucosal melanomas. The depth of invasion is difficult to measure because they are often biopsied in a fragmented way, but they usually are deep lesions, with depths often of ≥1 cm. They should be resected with wide margins if possible. Resection of melanomas of the nasopharynx, oropharynx, and sinuses is limited by the bony structures of the skull and the base of the brain. Vulvovaginal melanomas may be widely resected in many cases but may also be constrained by efforts to preserve urinary and sexual function. They may also be associated with extensive radial growth in addition to the invasive lesion, which can lead to multifocal local recurrences. Anorectal melanoma may usually be resected widely by an abdominoperineal resection, but this morbid operation is not associated with higher survival rates than local excision only.138 Adjuvant local radiation therapy may be of value when widely clear margins are not feasible.139 However, no randomized, prospective trials of radiation have been performed in this setting. SNBx has been performed for vulvovaginal melanomas, but its impact on ultimate clinical outcome is not known.140 It may also be performed for anorectal melanomas,141 but pelvic and systemic metastases are more concerning for ultimate outcome than the risk of groin metastases. SNBx is not generally feasible for mucous membrane melanomas of the head and neck because of technical considerations.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree