Pancreatic ductal adenocarcinoma (PDA) is the most common pancreatic malignancy comprising approximately 85% of cases. Many aspects of surgical resection of pancreatic cancer have been evaluated as to their effects on morbidity and mortality, including evaluation of anastomotic techniques, the role of extended lymphadenectomies, and the use of vascular reconstruction. Progress in the perioperative care of those undergoing pancreatic resection for PDA has resulted in improved outcomes. This review discusses the preoperative evaluation of a patient with pancreatic cancer and addresses the surgical management of these patients, with special attention to recent areas of progress and controversy.
Pancreatic ductal adenocarcinoma (PDA) is the most common pancreatic malignancy, comprising approximately 85% of cases. In 2009, it is estimated that more than 34,000 patients will die of PDA and the incidence closely matches this mortality rate, illustrating the aggressive nature of the disease, which is worsened by an often delayed diagnosis. Despite minor improvements in chemotherapeutic regimens, the median survival for patients diagnosed with PDA is 4 to 6 months. However, for the 10% to 20% of patients who are operative candidates at the time of diagnosis, the median survival, as reported in phase 3 trials, is 11 to 22 months. Many aspects of surgical resection of pancreatic cancer have been evaluated as to their effects on morbidity and mortality, including evaluation of anastomotic techniques, the role of extended lymphadenectomies, and the use of vascular reconstruction. In addition, progress in the perioperative care of those undergoing pancreatic resection for PDA has resulted in improved outcomes. This review discusses the preoperative evaluation of a patient with pancreatic cancer and addresses the surgical management of these patients, with special attention to recent areas of progress and controversy.
Clinical presentation
The retroperitoneal location of the pancreas creates a diagnostic challenge for the clinician regarding PDA. Because of this anatomic location, patients do not typically present with symptoms until their disease has progressed, often past the point where surgical therapy can offer a cure. Lesions in the tail of the pancreas are particularly difficult because any symptoms are usually due to invasion of contiguous structures or metastatic disease. Most patients with PDA present with lesions in the pancreatic head or neck (65%), with 15% present in the body/tail, and the remaining 20% being diffuse in nature. Symptoms of pancreatic cancer are often nonspecific ones such as weight loss and anorexia/malnutrition. Painless jaundice is a common presenting symptom, but occasionally symptoms are more ominous: abdominal pain, gastric outlet obstruction, and pruritus indicate potentially extensive tumor growth with biliary obstruction. In addition, new-onset diabetes is often diagnosed in 40% of patients concomitantly with their diagnosis of PDA or can be detected upward of 2 years before the diagnosis of PDA. The clinical manifestations of pancreatic cancer are therefore variable and often delayed in their presentation, creating a hazard for patients with the disease to receive timely treatment and a challenge to the clinician ( Table 1 ).
| Sign/Symptom | Incidence (%) |
|---|---|
| Weight loss | 50–90 |
| Pain | 75–80 |
| Malnutrition | 50–75 |
| Jaundice | 70 |
| Anorexia | 60 |
| Diabetes | 15–40 |
| Ascites | 5 |
| Gastric outlet obstruction | 5 |
Pancreatic adenocarcinoma staging
The staging of pancreatic cancer has undergone an evolution in the past 10 years that reflects a better understanding of the pathophysiology of the disease as well as better preoperative imaging and surgical techniques. At present, the tumor-node-metastasis (TNM) staging of the American Joint Commission on Cancer (AJCC) is employed ( Table 2 ). The current system, in its sixth version, emphasizes preoperative imaging to stratify patients who have invasion of the celiac axis or superior mesenteric artery and are therefore unable to undergo potentially curative resection. Invasion of the superior mesenteric vein or portal vein was once considered a locally advanced and unresectable disease according to earlier AJCC guidelines. However, as discussed later, this T3 disease no longer precludes patients from resection, as venous reconstruction in these patients can achieve survival rates similar to those without venous invasion. In addition, regional lymph node disease is categorized as being present or absent, without designation based on number of nodes involved as the fifth version of the AJCC staging system previously outlined. Finally, although there has been no change in the distinction between the absence or presence of distant metastatic disease (M0 vs M1), the overall staging has changed and incorporates a better understanding of prognosis for patients with pancreatic cancer. The current system therefore attempts to identify patients with metastatic disease (Stage IV) versus those who are potentially resectable (Stage I–III) based on high-resolution imaging.
| Primary Tumor (T) | Regional Lymph Nodes (N) | Distant Metastasis (M) | AJCC Stage |
|---|---|---|---|
| T0: No evidence of primary | N0: No nodal metastasis | Mx: Distant metastasis cannot be assessed | 0: Tis, N0, M0 |
| Tis: in situ | N1: Regional lymph node metastasis | M0: No distant metastasis | IA: T1, N0, M0 IB: T2, N0, M0 |
| T1: Limited to pancreas (≤2 cm) | M1: Distant metastasis | IIA: T3, N0, M0 IIB: T1-3, N1, M0 | |
| T2: Limited to pancreas (>2 cm) | III: T4, N0-1, M0 | ||
| T3: Extends beyond pancreas without CA or SMA involvement | IV: T1-4, N0-1, M1 | ||
| T4: Involves CA or SMA |
Pancreatic adenocarcinoma staging
The staging of pancreatic cancer has undergone an evolution in the past 10 years that reflects a better understanding of the pathophysiology of the disease as well as better preoperative imaging and surgical techniques. At present, the tumor-node-metastasis (TNM) staging of the American Joint Commission on Cancer (AJCC) is employed ( Table 2 ). The current system, in its sixth version, emphasizes preoperative imaging to stratify patients who have invasion of the celiac axis or superior mesenteric artery and are therefore unable to undergo potentially curative resection. Invasion of the superior mesenteric vein or portal vein was once considered a locally advanced and unresectable disease according to earlier AJCC guidelines. However, as discussed later, this T3 disease no longer precludes patients from resection, as venous reconstruction in these patients can achieve survival rates similar to those without venous invasion. In addition, regional lymph node disease is categorized as being present or absent, without designation based on number of nodes involved as the fifth version of the AJCC staging system previously outlined. Finally, although there has been no change in the distinction between the absence or presence of distant metastatic disease (M0 vs M1), the overall staging has changed and incorporates a better understanding of prognosis for patients with pancreatic cancer. The current system therefore attempts to identify patients with metastatic disease (Stage IV) versus those who are potentially resectable (Stage I–III) based on high-resolution imaging.
| Primary Tumor (T) | Regional Lymph Nodes (N) | Distant Metastasis (M) | AJCC Stage |
|---|---|---|---|
| T0: No evidence of primary | N0: No nodal metastasis | Mx: Distant metastasis cannot be assessed | 0: Tis, N0, M0 |
| Tis: in situ | N1: Regional lymph node metastasis | M0: No distant metastasis | IA: T1, N0, M0 IB: T2, N0, M0 |
| T1: Limited to pancreas (≤2 cm) | M1: Distant metastasis | IIA: T3, N0, M0 IIB: T1-3, N1, M0 | |
| T2: Limited to pancreas (>2 cm) | III: T4, N0-1, M0 | ||
| T3: Extends beyond pancreas without CA or SMA involvement | IV: T1-4, N0-1, M1 | ||
| T4: Involves CA or SMA |
Assessment for surgical resection and preoperative biliary drainage
Imaging Modalities
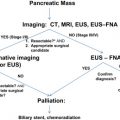
The use of preoperative imaging is a critical factor in the workup of patients with PDA. This imaging begins the staging process for the disease and influences the possibility of undergoing a surgical resection. The sensitivity of detecting PDA on a computed tomography (CT) scan has been reported to be 89% to 97% but decreases, as expected, with smaller pancreatic tumors. For tumors greater than 15 mm, Legmann and colleagues determined a sensitivity of 100% for CT scan compared with only 67% for tumors less than 15 mm. To acquire the most optimal images for the diagnosis of PDA, a dual-phase or “pancreatic protocol” CT should be used. With this protocol, images are acquired at 2 phases of peak contrast enhancement. The first is immediately after the arterial phase but before the hepatic phase of enhancement, referred to as the pancreatic phase, with the second phase correlating to the venous enhancement of the liver. The pancreatic phase allows optimal distinction between normal pancreatic parenchyma and a pancreatic mass while enabling evaluation of peripancreatic vessels to determine the extent of tumor involvement. The ability of CT to detect lymph node involvement is very poor based on size criteria alone, because enlarged lymph nodes may merely be reactive or normal-sized nodes may harbor micrometastasis. Enlarged peripancreatic lymph nodes should therefore not preclude a patient from surgery, as the ability of CT to determine vascular invasion is the single best predictor of resectability. The goal of the hepatic phase is to differentiate between normal hepatic parenchyma and hypovascular metastasis. Reports have indicated that up to 11% of pancreatic cancers are isoattenuating on the pancreatic and hepatic phases, making it potentially difficult to distinguish tumor from surrounding normal parenchyma unless other signs such as ductal dilatation or mass effect are present. Although magnetic resonance imaging (MRI) has not been demonstrated to be superior to CT scanning based on a meta-analysis showing a sensitivity of 84% for MRI compared with 91% for CT, selected cases such as evaluation of an isoattenuating tumor may show benefit in using MRI as an adjunct to a CT scan. Where MRI is perhaps most efficacious is in the evaluation of suspicious hepatic lesions that are less than 1 cm as seen on CT. CT is often unable to discriminate between benign and malignant lesions at this size but MRI has been shown to more accurately differentiate between the two. Although other technologies such as positron emission tomography, ultrasound, and endoscopic ultrasound are available and have been used in the evaluation of a patient with PDA or suspected PDA, their utility is limited to circumstances of diagnostic uncertainty or preparation for potential neoadjuvant therapy. In addition, newer MRI techniques may prove beneficial in the evaluation of pancreatic masses; however, in the absence of a contraindication for CT scan, such as contrast dye allergy, MRI is more costly and time consuming. Therefore, none of these alternative modalities have proved to be superior to pancreatic protocol CT in the determination of patients who are candidates for surgical resection.
Diagnostic Laparoscopy
As previously described, the ability of CT to accurately stage patients is limited by its inability to detect hepatic metastasis smaller than 1 cm or peritoneal metastasis. With laparoscopy continuously becoming more mainstream, surgeons responsible for treating patients with pancreatic cancer have adopted the technology to assist in clinically staging patients. The main source of debate relates to which patients should undergo laparoscopy, selective patients with advanced disease or all patients, to detect occult metastatic or advanced pancreatic cancer. However, with imaging technology constantly improving, the ability to pick up metastasis has also improved. This advance places the utility of preoperative laparoscopy into question. The morbidity associated with a diagnostic laparoscopy for staging of PDA is very low and ranges from 0% to 4%, with most literature reporting a mortality rate of 0%. Several prospective trials have not only reaffirmed the ability of laparoscopy to prevent a certain subset of patients from undergoing an unnecessary laparotomy but have also demonstrated it to be fiscally sound. In one of the earlier trials, Warshaw and colleagues evaluated 40 patients with histologically confirmed PDA who were appropriate surgical candidates based on preoperative evaluation. All 40 patients underwent laparoscopy and 14 demonstrated metastatic disease at the time of laparoscopy (35%). The remaining 26 patients had a negative laparoscopy but on subsequent laparotomy, the negative finding was confirmed in 23 (82% positive predictive value). Two of the false-negative cases were due to inadequate exposure during laparoscopy, while the third case resulted from the metastatic lesion located within the liver parenchyma discovered only during manual palpation. Therefore, 43% of the patients had metastatic disease not detected by CT scan, of which laparoscopy detected 82%. Unfortunately, this study was performed in an era before high-resolution helical CT scans. It is argued, therefore, that such numbers would not be duplicated if more modern CT scanners were used. In a more recent study by Maithel and colleagues a prospectively gathered database was retrospectively analyzed, and 491 patients were identified with PDA that was amenable to surgical resection by dual-phase CT scan or MRI. Patients with borderline resectable disease as identified by tumor extension to the celiac axis, abutments of the superior mesenteric artery, or short segment involvement of the portal or superior mesenteric veins were excluded, as were any patients with suspicion for distant metastasis. These 491 patients then underwent diagnostic laparoscopy with detailed examination of the liver, peritoneal surfaces, and transverse colon mesentery. Ninety-six patients were unresectable at the time of laparoscopy because of metastasis to the liver (62%), peritoneum (17%), or locoregional spread (22%). Seventy-two percent of patients were spared a nontherapeutic laparotomy based on the results of laparoscopy alone. Excluding patients who had locoregional spread, 92% of those with hepatic or peritoneal metastasis were spared a nontherapeutic laparotomy. Maithel and colleagues went on to determine if carbohydrate antigen (CA) 19-9 levels correlated with unresectability in this patient population. The median preoperative CA 19-9 level for those patients who were resected was 131 U/mL compared with 379 U/mL in those who had unresectable disease ( P <.003). Using multivariate regression analysis, a CA 19-9 value greater than 130 U/mL preoperatively was a predictor of tumor unresectability (hazard ratio 2.7). Some groups have sought to increase the sensitivity of laparoscopy for detecting unresectable PDA by performing a more extensive peripancreatic and periportal dissection or through the use of intraoperative ultrasound to detect occult hepatic metastasis. Finally, there are few studies to address the question of cost benefit from the use of staging laparoscopy. In general, use of staging laparoscopy has been found to be cost effective in selected cases and only when it will detect occult metastasis, thus avoiding an unnecessary laparotomy. To optimize the utility of staging laparoscopy for pancreatic cancer, it should follow a pancreatic protocol CT scan and be used only in selected cases of patients who are at high risk for metastatic disease, such as those with primary tumors greater than 4 cm, markedly elevated CA 19-9 levels, or equivocal findings of locally advanced or metastatic disease on CT scan.
Preoperative Biliary Drainage
Because the vast majority of pancreatic cancers are located in the pancreatic head, obstructive jaundice is a common presenting symptom. Therefore, preoperative biliary drainage is often employed to alleviate the symptoms of pruritus or in the setting of neoadjuvant therapy in which surgical treatment will be delayed. The use of preoperative biliary diversion was previously routinely performed following concerns about perioperative morbidity in a jaundiced individual undergoing a pancreaticoduodenectomy (PD). However, these studies are faulted by their retrospective nature and heterogeneous patient population. Modern literature does not corroborate these studies, and recent randomized trials have proven these concerns unfounded.
To ascertain whether preoperative biliary drainage or the process of preoperative biliary instrumentation alone is responsible for increased postoperative morbidity after PD, Povoski and colleagues evaluated their experience of 240 consecutive patients at Memorial Sloan-Kettering Cancer Center from 1994 to 1997 who underwent PD. Eighty percent of the PDs were performed for adenocarcinoma of the pancreas or periampullary region. Of the 240 patients, 175 (73%) underwent preoperative biliary instrumentation defined as cannulation of the biliary tract by surgical or nonsurgical techniques (percutaneous transhepatic cholangiography, endoscopic retrograde cholangiopancreatography, or common bile duct exploration). A cohort of 126 patients subsequently underwent biliary drainage preoperatively (endoscopic stents, percutaneous drains/stents, or surgical drainage procedure). The overall morbidity following PD was 48% (114/240) with a postoperative mortality of 5% (12/240). Overall infectious complications were seen in 34% (81/240) of patients while 14% (33/240) suffered from intra-abdominal abscess post-PD. Preoperative biliary stenting, but not instrumentation, was determined to be the only statistically significant factor associated with overall complications ( P = .025), infectious complications ( P = .014), intra-abdominal abscess ( P = .022), and postoperative death ( P = .037). Despite the heterogeneity of the patient population, an evaluation of patients who underwent PD for PDA or periampullary adenocarcinoma still determined that biliary drainage was the only statistically significant predictor of postoperative infectious complications ( P = .038), intra-abdominal abscess ( P = .001), and death ( P = .01).
In contrast, a study performed by Sohn and colleagues at the Johns Hopkins Medical Institutions evaluating preoperative biliary stenting in their population of patients who underwent PD did not reveal an overall increase in complication rates or in the rate of mortality in patients who underwent preoperative biliary drainage compared with patients who did not undergo preoperative stenting. This group retrospectively analyzed the data from 567 patients who underwent PD but had no previous history of biliary bypass. Of these people, 408 (72%) underwent preoperative biliary stenting, with 146 patients from this cohort (36%) having undergone an endoscopic approach. PD was performed for periampullary adenocarcinoma in 74% of the stented group and 57% of the unstented group ( P = .0001) with 47% and 38% of the pancreatic resections performed specifically for PDA, respectively. In comparing postoperative morbidity and mortality in the stented versus unstented groups, the only statistically significant difference was in the rates of wound infection and pancreatic fistula formation between the 2 groups ( Table 3 ). The overall complication rate (35% vs 30%, respectively) and mortality rate (1.7% vs 2.5%, respectively) between stented and unstented was not statistically significant. However, the rate of wound infection in the stented group was 10% compared with a 4% incidence in the unstented group ( P = .02), corroborating the findings of Povoski and colleagues. One additional finding of interest was the increased incidence of pancreatic fistula formation in the stented group (10%) versus the unstented group (4%), which was likewise statistically significant ( P = .02). However, this particular difference was not significant when evaluating patients who had periampullary adenocarcinoma (9% vs 3% in stented vs unstented, P = .07) perhaps reflecting an increased risk for individuals who underwent resection for chronic pancreatitis rather than adenocarcinoma, as this group of patients represented 11% and 15% of the stented and unstented groups, respectively.
| Author | N | Morbidity (%) | Mortality (%) | Infectious Complications (%) | Wound Infection (%) | Intra-abdominal Abscess (%) | Pancreatic Leak/Fistula (%) |
|---|---|---|---|---|---|---|---|
| Povoski | |||||||
| Total | 240 | 48 | 5 | 34 | 16 | 14 | 10 |
| Stented | 126 | 55* | 8* | 41* | n/a | 19* | n/a |
| Unstented | 114 | 39 | 3 | 25 | n/a | 8 | n/a |
| Sohn | |||||||
| Total | 567 | 34 | 1.9 | 29 | 8 | 4 | 8 |
| Stented | 408 | 35 | 1.7 | 32 | 10* | 4 | 10* |
| Unstented | 159 | 30 | 2.5 | 22 | 4 | 6 | 4 |
| Pisters | |||||||
| Total | 265 | 87 | 1 | 35 | 10 | 8 | 8 |
| Stented | 172 | 88 | 1 | 37 | 13* | 6 | 8 |
| Unstented | 93 | 86 | 1 | 31 | 4 | 11 | 10 |
| Hodul | |||||||
| Total | 212 | 64 | 2 | 26 | 5.7 | 6 | 11 |
| Stented | 154 | 33 | 2 | 29 | 8* | 7 | 10 |
| Unstented | 58 | 43 | 2 | 21 | 0 | 5 | 14 |
| Sewnath | |||||||
| Total | 290 | 51 | 1 | 35.5 | 7.6 | 15.5 | 12.4 |
| Stented | 232 | 50 | 1.3 | 37 | 7.3 | 15.5 | 13.8 |
| Unstented | 58 | 55 | 0 | 31 | 8.6 | 15.5 | 6.9 |
Pisters and colleagues from the MD Anderson Cancer Center similarly evaluated their experience of 300 patients who underwent PD for malignancy or suspected malignancy, with 60% of patients having PDA on final histology. A total of 93 patients (31%) did not undergo any preoperative biliary decompression compared with 172 patients (57%) who did. Of this cohort, the drainage procedure consisted of 81% endobiliary stents, 16% percutaneous transhepatic catheter, and 2% undergoing other forms of biliary drainage (T-tube, Wallstent, or cholecystostomy tube). Despite a difference in wound infection rates between patients undergoing preoperative biliary stenting and those who did not (13% vs 4%, respectively, P = .028), there was no statistically significant difference between the groups when compared with overall complications, major complications, anastomotic leak, or perioperative death. Despite the increase in wound infection seen in these 3 studies, several prospective randomized trials of preoperative biliary stenting have not shown such a difference. A prospective randomized multicenter trial is currently accruing patients and will hopefully offer better insight into the controversial topic of preoperative biliary stenting. Until that time, preoperative biliary diversion should likely be reserved for patients who will undergo neoadjuvant therapy for pancreatic cancer and thus have a delayed surgical intervention, or for those with severe symptoms who also will have a delay in surgical treatment, being cognizant that a slightly increased risk of postoperative wound infections may be present.
Metallic versus Plastic Biliary Stents
Additional debate exists over the type of material used for a biliary stent, as metal stents have a longer patency rate that is ideal in patients who are not surgical candidates, and therefore offer a more definitive option for biliary decompression during chemotherapy treatment. In addition, the longer patency of metal stents would benefit those undergoing neoadjuvant therapy for PDA to avoid the complications of stent occlusion and the necessity of having a plastic stent frequently changed. However, earlier practices favored plastic stents in those who would undergo definitive surgery, as it was thought that metal stents created an inflammatory reaction that made the dissection during PD difficult. These claims have proved unfounded, as the preoperative use of expandable metal stents before PD has not been shown to significantly increase operative time, operative blood loss, or perioperative mortality. For these reasons expandable metallic stents may be an appropriate means of preoperative biliary decompression in the appropriate patient who will undergo PD. Otherwise, the clinician must understand the risk of biliary stent complications in those patients who have a plastic stent in place, most often related to stent occlusion and cholangitis.
Surgical techniques
History and Overview of Surgery for Pancreatic Cancer
PD was first described in 1912 by Walter Kausch and later reformed by Allen O. Whipple in 1935 to consist of a 2-stage PD whereby biliary diversion and a gastrojejunostomy were performed during the first stage, followed 3 to 4 weeks later by pancreatic head and duodenal resection. Whipple eventually modified the PD into a 1-stage procedure that now bears his name. Despite years of technical advancements as well as advancements in pre- and postoperative management, the surgical goals remain the same. Surgical treatment of pancreatic cancer aims to resect all gross and microscopic evidence of disease and subsequently reestablish enteric continuity.
The approach used to resect pancreatic cancer depends on the location of the tumor. For example, a lesion in the tail of the pancreas necessitates a distal pancreatectomy. Because approximately 60% to 70% of pancreatic cancers arise from the head of the pancreas, the most common operation performed to resect these tumors is a PD. Pancreatic cancers that arise in the body of the pancreas often are locally advanced at the time of diagnosis and are unable to be resected. However, if resection is possible, the anatomic position of the tumor dictates the extent of resection, with lesions nearer to the pancreatic tail being treated with a distal pancreatectomy and those closer to the head with an extended pancreatiocoduodenectomy. With this measure as much pancreatic parenchyma as possible can be preserved to limit postoperative diabetes. Central pancreatectomies have been performed for premalignant or low-grade (T1) tumors, but is rarely employed for patients with PDA due to concerns regarding lymph node clearance.
Technique of Pancreaticoduodenectomy
After appropriate preoperative workup as described previously, a PD is performed in several discrete steps. After entry into the abdomen is established with either a midline laparotomy or bilateral subcostal incision (Chevron incision), the liver and peritoneal surfaces are inspected to assess for metastatic disease that may not have been detected on preoperative imaging. A diagnostic laparoscopy, if performed, would likewise satisfy this criterion to rule out previously undetected Stage IV disease before laparotomy as described previously. The dissection begins by incising the posterior peritoneum of the duodenal C-loop and mobilizing the hepatic flexure such that the duodenum and transverse mesocolon are separated. A Kocher maneuver is then performed to mobilize the duodenum and pancreatic head to the level of the superior mesenteric vein (SMV) anteriorly and left renal vein posteriorly. Finally, the ligament of Treitz can be incised behind the superior mesenteric vessels, which facilitates the exposure of the distal duodenum and uncinate process of the pancreas. Attention is next directed to the portal dissection, which begins with a cholecystectomy. The common bile duct is circumferentially isolated and the common hepatic duct is divided just above the junction with the cystic duct, and the distal common bile duct is dissected to the pancreatic head. The gastroduodenal artery is then identified and ligated, and the avascular plane between the anterior portal vein (PV) and posterior pancreas is initiated above the level of the duodenum. Next, the SMV is identified by entering the lesser sac via separation of the greater omentum from the transverse mesocolon. The middle colic vein is then followed to the inferior border of the pancreas where the posterior peritoneum is divided and the SMV is encountered. Dissection is performed anterior to the SMV and posterior to the pancreas, eventually connecting with the previous anterior PV/posterior pancreas dissection. Once this stage is completed, the stomach is divided (or duodenum if pylorus preservation is desired) as well as the jejunum approximately 8 to 10 cm distal to the ligament of Treitz. This action is followed by division of the pancreas, which proceeds along the previously created dissection plane traveling from the SMV to the SMV/PV confluence. Most critical for oncologic purposes is the separation of the divided pancreatic head from the SMV and superior mesenteric artery (SMA), which is performed next. The SMA is mobilized to its origin and the pancreatic head is separated from the right lateral wall of the SMA by serial ligation and division of the tissue, attaching the uncinate to the SMA. Once completed, the specimen is removed and the retroperitoneal margin is marked for the pathologist. Frozen sections of the transected pancreatic and common bile duct margins are performed to ensure an R0 resection. If this is not confirmed, then additional excision is needed. At this time, reconstruction is performed by creating the pancreaticojejunostomy/pancreaticogastrostomy, choledochojejunostomy, and gastrojejunostomy/duodenojejunostomy. Some surgeons place a feeding jejunostomy, as some patients may develop delayed gastric emptying or may need their caloric intake augmented postoperatively.
Traditional versus Pylorus-Preserving Pancreaticoduodenectomy
Much debate has existed regarding the optimal strategy for gastrointestinal reconstruction after PD since Watson first reported the preservation of the entire stomach and duodenum with reconstruction via a duodenojejunostomy in 1944, and the modern popularization of the procedure by Traverso and Longmire. Proponents of the pylorus-preserving PD argue that it offers better digestion, with prevention of marginal ulceration at the anastomosis. Opponents of the procedure raise concerns that nodal clearance of the suprapyloric and infrapyloric perigastric nodes may be limited. Several randomized trials have been conducted comparing such outcomes as delayed gastric emptying, resection margins, and overall survival ( Table 4 ).
| Author | N | Morbidity (%) | Operative Mortality (%) | R0 Resection (%) | Median Survival (Months) | DGE (%) | Pancreatic Leak/Fistula (%) |
|---|---|---|---|---|---|---|---|
| Tran | |||||||
| Total a | 170 | 58 | 5 | 78 | n/a | 22 | 13.5 |
| SP | 83 | 61 | 7 | 83 | 11 | 23 | 14 |
| PPP | 87 | 54 | 3 | 74 | 12 | 22 | 13 |
| Seiler | |||||||
| Total | 130 | 62 | 2.3 | 79 | 19.3 | 38.5 | 2.3 |
| SP | 66 | 68 | 3 | 72 | 18.2 | 45 | 2 |
| PPP | 64 | 55 | 2 | 86 | 19.2 | 31 | 3 |
| Lin | |||||||
| Total | 36 | n/a | 8 | n/a | 16 | 17 | 5.5 |
| SP | 22 | n/a | 9 | n/a | 12 | 0 | 4.5 |
| PPP | 14 | n/a | 7 | n/a | 33 | 43* | 7 |
| Paquet | |||||||
| Total | 40 | 52 | 2.5 | 100 | n/a | 17.5 | 7.5 |
| SP | 23 | 48 | 4 | 100 | n/a | 4 | 9 |
| PPP | 17 | 59 | 0 | 100 | n/a | 35* | 6 |
| Roder | |||||||
| Total b | 110 | 51 | 1.8 | 47 | 12 | 8 | 24.5 |
| SP | 62 | 40 | 1.6 | 45 | n/a | 0 | 21 |
| PPP | 48 | 65 | 2.1 | 50 | n/a | 19* | 29 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree