The introduction of all -trans retinoic acid to anthracycline-based chemotherapy has revolutionized the prognosis of patients with acute promyelocytic leukemia (APL). The introduction of arsenic trioxide enabled the therapeutic approach of rationally targeted frontline protocols with minimal or no traditional cytotoxic chemotherapy and without compromise of previously established outstanding outcomes with anthracycline-based regimens. Although most of the current investigative efforts in APL are focused on developing potentially curative therapy without the exposure to toxicities and risks of DNA-disrupting agents, the cure rate can further be increased by implementing meticulous supportive care strategies that counter early coagulopathy-related deaths.
Acute promyelocytic leukemia (APL) is a distinct morphologic variant of acute myeloid leukemia (AML), accounting for approximately 10% to 15% of the adult cases of AML diagnosed in the United States annually. The leukemia cells are usually easy to distinguish morphologically from others and are characterized by a specific reciprocal translocation t(15;17), which fuses the PML (promyelocyte) gene from chromosome 15 to the RAR-α (retinoic acid receptor-α) gene of chromosome 17. Consistently found in all cases of t(15;17) APL, the resulting PML-RARα fusion gene on der(15) encodes a chimeric transcript of the 2 DNA-binding domains that shows altered transcriptional regulatory properties, eventually leading to the block of retinoic-acid–induced myeloid differentiation.
Ever since the seminal description by Hillestad in 1957, APL has been well recognized as one of the most fatal forms of acute leukemia at presentation or during induction, primarily because of an associated complex and often catastrophic bleeding disorder. However, the introduction of all- trans retinoic acid (ATRA) in 1985, combined with anthracycline-based chemotherapy, has revolutionized the prognosis of this disease, with unprecedented complete response (CR) rates in excess of 90%, and cure rates of approximately 80%. The pharmacologic concentrations of ATRA used in the treatment of APL lead to dissociation of N-CoR (nuclear corepressor) (a ubiquitous nuclear protein that mediates transcriptional repression) presumably permitting differentiation of the leukemic cells. As a result, the long-term outcome in most patients with APL is favorable. Nevertheless, a small percentage of patients have a variant form of the disease with different fusion transcripts such as PLZF/RAR-α or STAT5b/RAR-α coding for fusion proteins that are almost invariably resistant to initial treatment with ATRA, necessitating the accurate baseline identification of candidates who can be expected to benefit from induction therapy with ATRA. FLT-3 mutations, particularly the internal tandem duplication (ITD) mutations, are also common in APL and seem to be present in approximately 40% of patients. These variants are often also characterized by the high white blood cell (WBC) count and the bcr3 PML breakpoint. Several retrospective studies have reported that the subgroup of patients with FLT-3 mutation have a higher death rate during induction chemotherapy without a significant difference in relapse rate or 5-year overall survival (OS). Nevertheless, there have been reports that through univariate analysis showed higher relapse and lower survival rates in patients with an FLT3-ITD.
The subsequent introduction of arsenic trioxide (ATO) further redefined the course of APL from a highly fatal to the most curable subtype of AML in adults. After several studies reported that the combination of ATRA and ATO is a highly effective and potentially curative treatment, an exciting new treatment strategy has emerged, which aims to eliminate exposure to conventional cytotoxic chemotherapy in treatment of many, if not most, patients with APL. Despite the advances in molecular biology and treatment approaches that have led to substantially decreased relapse rates, even among high-risk patients, the persistent challenge of the complex and life-threatening coagulopathy before and during induction therapy has remained the principal cause of early death and has emerged as the major cause of treatment failure among patients with APL. Other questions of importance in APL are centered on defining the best treatment of patients with high-risk disease, the role of ATO in initial therapy, and the roles of maintenance therapy and molecular monitoring. Nevertheless, implementing rapid, aggressive, and comprehensive strategies that mitigate early death has become one of the most important goals in APL treatment and is sure to further increase the cure rate of APL.
In this review, the therapeutic approaches that have led to the current frontline treatment in APL are summarized, focusing on development of new and rationally targeted therapeutic approaches that aim to eliminate toxicities of conventional chemotherapy without compromising cure rates; the importance of strategies to further increase the cure rate of APL by addressing early hemorrhagic deaths is also highlighted.
Induction therapy
In the age preceding the therapeutic use of ATRA, APL was treated much like other subtypes of AML, with the standard induction regimens based on an anthracycline and cytarabine (Ara-C) yielding 70% CR rates among newly diagnosed patients. However, even among those patients who initially achieved CR, between 50% and 65% subsequently relapsed, whereas only 30% to 50% remain alive at 2 years.
Initial Studies of ATRA in APL
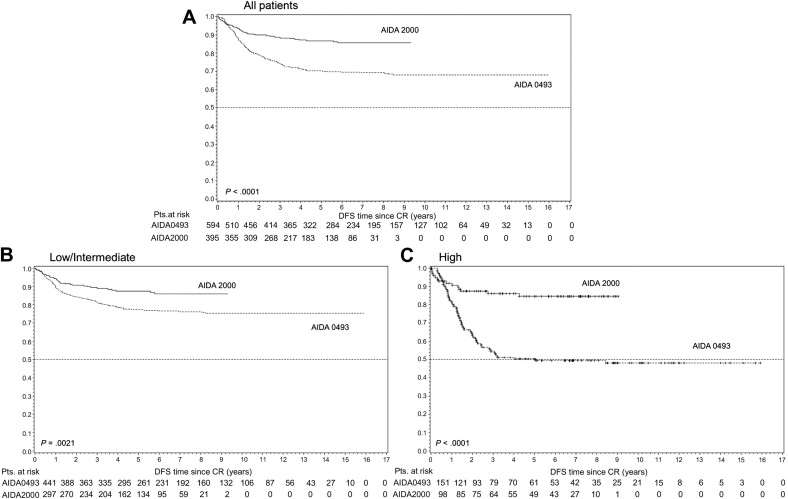
The evolution of creative treatment approaches for APL was spurred by an initial report, which documented CR rates of 85% with ATRA as a single induction agent. Among the first large studies to explore the role of ATRA either as a single agent or in combination with chemotherapy, the first North American Intergroup study I0129/E2491 reported an equivalent remission rate of 70% with single-agent ATRA compared with induction with Ara-C and daunorubicin. These encouraging results were tempered by studies of ATRA as a single agent that reported relapse of these patients from CR without further chemotherapy. As a result, subsequent trials such as that conducted by the European APL (EAPL) group focused on improving the clinical outcome through a combination of ATRA and chemotherapy. These investigators reported equal remission rates of 92% among the groups randomized to induction with concurrent therapy with ATRA plus chemotherapy with Ara-C and daunorubicin, versus sequential ATRA followed by chemotherapy. However, a reduced relapse rate at 2 years (6% vs 16%) was observed in the arm with concurrent versus sequential therapy. After validation in multiple large multicenter trials ( Table 1 ), these results led to the simultaneous administration of ATRA and anthracycline-based chemotherapy as the current standard induction approach in newly diagnosed patients with APL, with outstanding disease-free survival (DFS) rates ( Fig. 1 ).
| Groups (References) | Year | N | CR (%) | D(E)FS (%) | Therapy |
|---|---|---|---|---|---|
| EAPL group | 1999 | 99 | 92% | 84 | ATRA + DA |
| GIMEMA | 1997 | 240 | 95 | 79 | ATRA + IDA |
| North American study | 1997 | 172 | 72 | 75 | ATRA-based induction/maintenance |
| PETHEMA | 1999 | 123 | 89 | 92 | ATRA + IDA (Ara-C, etoposide in consolidation) |
| GAMLCG | 2000 | 51 | 92 | 88 | HiDAC + ATRA |
Choice of Anthracyclines
There is no clear evidence that one anthracycline is superior to another when combined with ATRA because no prospective randomized trials have directly compared daunorubicin and idarubicin in the ATRA era. The best available evidence stems from the pre-ATRA era. Even though it seems to suggest that idarubicin is associated with an improved outcome compared with daunorubicin or amsacrine, there is no distinct superiority between the two because both seem equally effective in APL.
Role of Ara-C in Initial Therapy
The question of the importance of the addition of Ara-C to anthracyclines in the induction regimen has been addressed with disparate results. The cooperative groups Gruppo Italiano Malattie Ematologiche dell’Adulto (GIMEMA) and the Programa Español de Tratamientos en Hematología (PETHEMA) omitted Ara-C during induction in nonrandomized prospective clinical trials, showing that ATRA plus idarubicin (AIDA) is as effective in inducing CR as a regimen containing Ara-C, with CR rates of 89% to 95% that were not influenced by the presenting WBC count. The National Cancer Research Institute (NCRI) in the United Kingdom (UK) reported no overall difference in response, relapse, or survival rates between the arms randomized to AIDA and ATRA plus daunorubicin and Ara-C. However, they did observe less myelosuppression without the use of cytarabine. In a contrasting report, the EAPL group (APL 2000) in a randomized study observed a statistically significant increase in relapse risk (13.4% vs 29%) and OS (92.9% vs 83.3%) without the addition of Ara-C in both induction and consolidation regimens. Despite the discrepancy of these reports, which can be attributed possibly to the choice and cumulative doses of anthracyclines, as well as differences in the consolidation regimens and the number of total consolidation courses, the choice of induction therapy is defined by the ability to tolerate anthracyclines and by whether the patient had high-risk or low-risk disease based on the WBC count at diagnosis. It seems that Ara-C can be omitted from ATRA plus anthracycline-based induction regimen in most patients, even those considered high-risk (WBC count >10,000/μL). The optimal anthracycline has not been determined. However, idarubicin seems to be sufficient to induce CR in almost all patients who survive the initial risk of early death during induction.
Consolidation therapy
Unlike the aim of induction therapy, which focuses on reduction of the total disease burden to less than the cytologically detectable level of approximately 10 9 cells, the goal of consolidation therapy in APL is to eliminate the remaining malignant leukemic stem cells that through replicative resilience lead to eventual relapse. Induction failure of any subgroup of patients with APL is generally related to bleeding, the APL differentiation syndrome, or infection and not disease progression. There is essentially no primary resistance in APL. Although ATRA-based induction therapy has a greater than 90% CR rate in APL, patients are invariably destined to relapse, necessitating effective consolidation therapy that provides durable remission. The benefit of adding ATRA in induction in improving DFS and OS by reducing relapse has been amply reported. Although not shown in any randomized trial, consecutive trials by the GIMEMA and PETHEMA groups have shown improvement in outcome by the addition of ATRA in consolidation. The optimal choice and intensity of consolidation regimens remain controversial, because many of the investigative efforts of the past decade have promoted the development of risk-adapted approaches that guide the treatment intensity as defined by the risk factors predictive of relapse.
Risk-adapted Approach to Consolidation Therapy
The necessity for a risk-adapted approach is reflected in the higher mortality of 19% in patients in CR older than 70 years, compared with mortality of 1% in those younger than 60 years. The currently used risk model stems from the 2 PETHEMA trials, which have identified 3 different groups of patients at risk for relapse: high-risk defined by presenting WBC counts 10,000/μL or greater and platelet count less than 40,000/μL; intermediate-risk with WBC count less than 10,000/μL and platelet count less than 40,000/μL and a low-risk with WBC count less than 10,000/μL and platelet count greater than 40,000/μL.
The results of the LPA99 trial of significantly improved outcome with the inclusion of ATRA in consolidation treatment were confirmed by the new AIDA-2000 trial of the Italian GIMEMA group. The PETHEMA group study (LPA2005) randomized high-risk patients younger than 60 years to receive Ara-C combined with ATRA and idarubicin in the first and third consolidation courses, reporting significantly lower relapse rate of 11% at 3 years (this is compared with 26% relapse rate in the historical cohort in the LPA99 trial, P = .03). More importantly, the study also investigated reducing the overall toxicity in low-risk and intermediate-risk patients by lowering the dose of mitoxantrone for the second consolidation course and eliminating Ara-C during the 3 consolidation courses. This treatment resulted in a comparable clinical outcome but with a significant reduction of toxicity (as reflected in the duration of neutropenia and thrombocytopenia) and a shorter overall hospital stay (17 days vs 22 days in the LPA99 trial; P <.001). The GIMEMA group (AIDA2000), which also included ATRA as a part of the consolidation regimen, similarly reported no difference in OS and a significant reduction of infections (40% vs 80%) and prolonged neutropenia with improved DFS at 6 years (85.9% vs 76.6% in the AIDA0493) for the low-risk and intermediate-risk patients who had Ara-C omitted from their consolidation courses. An additional study conducted by the UK NCRI failed to show any benefit of Ara-C regardless of risk stratification. With the goal of further reduction in toxicity, the North American Intergroup Trial C9710 randomized patients after CR to receive either 2 courses of 25 days of ATO (5 days a week for a total of 5 weeks) followed by a standard postremission regimen with 2 courses of ATRA plus daunorubicin, or postremission therapy alone. Treatment with ATO, particularly in the high-risk subgroup, showed significantly better event-free survival (EFS) and OS. This finding represents an attractive alternative among those patients with a high-risk disease who may be in particular need of a less cardiotoxic consolidation regimen.
Given the overall body of evidence, it seems that high-risk patients benefit from either the addition of intermediate-dose Ara-C to consolidation or ATO as an early consolidation course with synergistic antileukemic effect of combination therapy.
Consolidation therapy
Unlike the aim of induction therapy, which focuses on reduction of the total disease burden to less than the cytologically detectable level of approximately 10 9 cells, the goal of consolidation therapy in APL is to eliminate the remaining malignant leukemic stem cells that through replicative resilience lead to eventual relapse. Induction failure of any subgroup of patients with APL is generally related to bleeding, the APL differentiation syndrome, or infection and not disease progression. There is essentially no primary resistance in APL. Although ATRA-based induction therapy has a greater than 90% CR rate in APL, patients are invariably destined to relapse, necessitating effective consolidation therapy that provides durable remission. The benefit of adding ATRA in induction in improving DFS and OS by reducing relapse has been amply reported. Although not shown in any randomized trial, consecutive trials by the GIMEMA and PETHEMA groups have shown improvement in outcome by the addition of ATRA in consolidation. The optimal choice and intensity of consolidation regimens remain controversial, because many of the investigative efforts of the past decade have promoted the development of risk-adapted approaches that guide the treatment intensity as defined by the risk factors predictive of relapse.
Risk-adapted Approach to Consolidation Therapy
The necessity for a risk-adapted approach is reflected in the higher mortality of 19% in patients in CR older than 70 years, compared with mortality of 1% in those younger than 60 years. The currently used risk model stems from the 2 PETHEMA trials, which have identified 3 different groups of patients at risk for relapse: high-risk defined by presenting WBC counts 10,000/μL or greater and platelet count less than 40,000/μL; intermediate-risk with WBC count less than 10,000/μL and platelet count less than 40,000/μL and a low-risk with WBC count less than 10,000/μL and platelet count greater than 40,000/μL.
The results of the LPA99 trial of significantly improved outcome with the inclusion of ATRA in consolidation treatment were confirmed by the new AIDA-2000 trial of the Italian GIMEMA group. The PETHEMA group study (LPA2005) randomized high-risk patients younger than 60 years to receive Ara-C combined with ATRA and idarubicin in the first and third consolidation courses, reporting significantly lower relapse rate of 11% at 3 years (this is compared with 26% relapse rate in the historical cohort in the LPA99 trial, P = .03). More importantly, the study also investigated reducing the overall toxicity in low-risk and intermediate-risk patients by lowering the dose of mitoxantrone for the second consolidation course and eliminating Ara-C during the 3 consolidation courses. This treatment resulted in a comparable clinical outcome but with a significant reduction of toxicity (as reflected in the duration of neutropenia and thrombocytopenia) and a shorter overall hospital stay (17 days vs 22 days in the LPA99 trial; P <.001). The GIMEMA group (AIDA2000), which also included ATRA as a part of the consolidation regimen, similarly reported no difference in OS and a significant reduction of infections (40% vs 80%) and prolonged neutropenia with improved DFS at 6 years (85.9% vs 76.6% in the AIDA0493) for the low-risk and intermediate-risk patients who had Ara-C omitted from their consolidation courses. An additional study conducted by the UK NCRI failed to show any benefit of Ara-C regardless of risk stratification. With the goal of further reduction in toxicity, the North American Intergroup Trial C9710 randomized patients after CR to receive either 2 courses of 25 days of ATO (5 days a week for a total of 5 weeks) followed by a standard postremission regimen with 2 courses of ATRA plus daunorubicin, or postremission therapy alone. Treatment with ATO, particularly in the high-risk subgroup, showed significantly better event-free survival (EFS) and OS. This finding represents an attractive alternative among those patients with a high-risk disease who may be in particular need of a less cardiotoxic consolidation regimen.
Given the overall body of evidence, it seems that high-risk patients benefit from either the addition of intermediate-dose Ara-C to consolidation or ATO as an early consolidation course with synergistic antileukemic effect of combination therapy.
Maintenance (postconsolidation) therapy
The response to completed consolidation therapy is routinely assessed using reverse transcriptase-polymerase chain reaction (RT-PCR) in the bone marrow, the goal being complete molecular remission (MR). The best therapeutic approach to maintenance therapy was controversial even in the pre-ATRA era, and despite the 2 randomized controlled trials that have reported a substantial benefit with ATRA-based maintenance therapy, its systematic use remains controversial. This situation is particularly true for those patients considered nonhigh risk, or those who achieve MR after consolidation treatment. Moreover, parallel to evolution of not only induction but also consolidation regimens that have incorporated ATO into first-line treatment of APL, the significance of maintenance therapy may have been reduced even further.
The North American Intergroup study I0129/E2491 reported a superior 5-year DFS (61% vs 36%), with a reduction in relapse rate (22% vs 39%), proving clinical benefit with ATRA-based maintenance therapy. The APL93 trial similarly confirmed the beneficial effect of adding ATRA to maintenance therapy in a randomized study in which the regimen consisting of continuous low-dose 6-mercaptopurine (6-MP), methotrexate (MTX), and intermittent ATRA showed the lowest relapse rate (8%) at 2 years, compared with relapse for 6-MP plus MTX (13%), ATRA alone (21%) versus no maintenance (35%). More importantly, this group recently updated the results of their previous study to indicate that the clinical benefit of maintenance therapy was mainly evident in high-risk patients whereas the low-risk and intermediate-risk patients experienced only marginal benefit. With contrasting results, randomized trials by the GIMEMA and Japanese Adult Leukemia Study Group (JALSG) have reported no difference in DFS with postconsolidation therapy, with follow-up of up to 12 years. However, the observations of the JALSG group trial may not entirely be applicable, because ATRA was not included in maintenance therapy. Furthermore, data from the AIDA study appeared to suggest that patients who test molecularly negative after the consolidation therapy experience no real benefit from maintenance therapy.
These studies differed significantly in the treatment approach. Both the JALSG and the GIMEMA groups administered a higher number of consolidation cycles with idarubicin as a choice of anthracycline therapy for induction and consolidation chemotherapy; in contrast, the EAPL and North American Intergroup trials administered only 2 consolidation courses with daunorubicin. Moreover, whereas studies by the GIMEMA and JALSG groups may have been confounded because all study patients tested negative for PML/RARα at the end of consolidation, the North American Intergroup and EAPL studies did not examine the MR status at the end of consolidation, raising a question whether the benefit that was observed by the maintenance therapy in their study largely reflected the response of patients with residual disease after consolidation treatment.
Overall, the sum of reported studies points to discrepancies that suggest that the benefit of maintenance treatment may be dependent on preceding induction and consolidation therapy, as well as PML/RARα molecular status after consolidation, which has clearly been shown to correlate with the relapse risk. Together with RT-PCR, which should be completed to document postconsolidation MR, subsequent molecular monitoring of patients can be performed based on the perceived risk of relapse. Furthermore, studies suggest that low-risk and intermediate-risk patients and those who achieve complete MR after consolidation may not benefit from the combination maintenance therapy. However, for high-risk patients, maintenance therapy for 1 to 2 years with intermittent ATRA and low-dose chemotherapy with 6-MP and MTX is recommended. Furthermore, molecular monitoring every 3 months for 2 years during maintenance for high-risk subgroups is also recommended, with the aim of monitoring and identifying potential molecular relapse. Because there are no prospective trial data in comparing 1 versus 2 years of maintenance therapy, we recommend continuation of maintenance therapy for 2 years unless toxicity develops. In those patients who develop cytopenias and have a negative molecular test with RT-PCR, a marrow analysis is recommended for evaluation of new cytogenetic anomalies that may represent therapy-related myelodysplastic syndrome (MDS) or AML.
Minimal residual disease monitoring
Minimal residual disease (MRD) monitoring entails identification of the molecular product of the chimeric PML-RARα transcript. The development of the real-time quantitative PCR (RQ-PCR) allowed more precise assessment of the kinetics of treatment response and relapse, setting foundation to MRD-directed therapy. Taking into account that frank relapse in APL can be complicated by a major risk of death because of hemorrhagic complications, and evidence that impending relapses can be reliably predicted by MRD monitoring with RT-PCR, there has been a growing interest inadopting MRD monitoring as a tool to guide and deliver preemptive therapy. Nevertheless, the optimal use of sequential MRD monitoring in treatment of APL remains controversial because of limited prospective data.
A prospective study by the PETHEMA group reported improved survival in patients salvaged with ATRA and anthracycline-based chemotherapy in molecular relapse compared with those who were treated only at frank hematologic relapse (historical control population). However, OS of 44% at 2 years for the historical cohort patients was considerably lower than what is currently achieved with ATO-based regimens. The UK NCRI prospective study has similarly reported a benefit from MRD-directed preemptive therapy using ATO, showing the evidence for MRD monitoring being far superior to presenting WBC count as a therapy guide and the strongest predictor of relapse-free survival (RFS) with less toxicity and reduced rates of frank relapses. However, there remain many unanswered questions from the trial. Given that half of the trial patients (MRC AML15) received maintenance after consolidation treatment, the difference in kinetics of PML-RARα transcripts and time from molecular to hematologic relapse among the group that did and did not receive maintenance therapy is unclear; furthermore, although the key aim of monitoring was to trigger preemptive therapy, more than half of the patients failed to have the salvage therapy initiated because of various technical reasons, questioning the large-scale practicality of this approach; the clinical outcome of patients in this trial reported in the 5-year RFS of 35% and OS of 41% remains less than optimal, implying that primary focus should be placed on improving the initial therapy in high-risk patients to prevent against, rather than detect, disease relapse.
The limited prospective data and the improved antileukemic efficacy of currently available standard treatments together question the true benefit of systematic molecular monitoring after consolidation therapy. As a result, the role of MRD monitoring as part of currently standard practice is changing, particularly for low-risk and intermediate-risk patients. The main reasons are the unanswered questions that pertain to the cumulative cost of serial testing, the implications of positive MRD screen, the chance of successful treatment at the time of MRD versus at the time of hematologic relapse as well as persistent anxiety potentially conferred by frequent testing.
Rigorous sequential RQ-PCR monitoring has been found to be the strongest predictor of RFS in APL and, when coupled with preemptive therapy, represents a valid strategy toward reducing rates of clinical relapse. Low-risk and intermediate-risk patients have a more favorable outcome with extremely low risk of relapse with currently available treatment protocols. Consequently, serious doubts can be raised about the usefulness of molecular monitoring in this group of patients if they are treated with contemporary strategies and followed with routine complete blood counts. On the other hand, the high-risk patients carry a relapse risk of approximately 10%, which seems to suggest there may be a greater benefit for sequential MRD monitoring in this patient population ; the optimal schedule for monitoring is still the subject for future research. Molecular monitoring may guide an appropriate salvage therapy for these patients based on the available evidence that suggests a higher relapse rate after a PCR-positive autologous hematopoietic stem cell transplantation (HSCT). In addition, a small subset of the high-risk patients may have the propensity for a central nervous system relapse. After achieving second morphologic remission, these patients are strongly recommended to receive intrathecal chemotherapy. Allogeneic transplantation should be reserved for those patients who have evidence of persistent disease despite salvage therapy.
Keeping in mind the pace at which new effective therapeutic strategies are evolving with less residual disease to detect, MRD monitoring in APL may soon become less important for most patients. Nevertheless, although still advised in the high-risk patients with APL, it is highly recommended that it be performed in reference laboratories with extensive expertise.
Role of HSCT
Those patients who experience persistence of disease after appropriate initial therapy or fail to achieve second molecular CR after salvage ATO at first relapse should be considered for allogeneic HSCT. Patients who relapse after initial therapy and achieve a second molecular CR after ATO salvage therapy do well with autologous HSCT. The choice between autologous versus allogeneic HSCT in second molecular CR was initially answered in a study that showed that for those patients with APL in second CR, autologous bone marrow transplantation (ABMT) with PML/RAR-α–negative marrow cells is likely to result in prolonged clinical and MRs. Conversely, patients who tested PCR-positive after reinduction required the use of alternative aggressive approaches, including unrelated allogeneic HSCT. This finding was also further investigated by the EAPL group, who showed an OS rate of 75% for patients with PCR-negative autologous HSCT, versus 52% for those who underwent allogeneic HSCT. Differences in the survival were likely reflective of cumulatively higher treatment-related mortality for the allogeneic HSCT arm. Allogeneic and autologous stem cell transplantations (SCTs) were directly compared in the same retrospective study, which concluded that if performed in MR, ABMT is effective in patients who relapse after initial treatment with ATRA. At the same time, allogeneic SCT was found to be associated with high treatment-related mortality when performed after salvage with intensive chemotherapy. Salvage with ATO instead, which has lower toxicity, promises to further improve the outcome of relapsing patients with APL.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree