The prognostic factors in adult acute leukemia have undergone a major change over the past decade and are likely to be further refined in the coming years. Age is the single most important prognostic factor in both acute myeloid leukemias and in acute lymphoblastic leukemias. Recurring cytogenetic abnormalities and molecular markers have become crucial for the prognosis of patients and for new directions in the development of targeted therapies. No less important is the development of a personalized approach to therapy as determined by the response to therapy using increasingly sensitive technologies.
Acute myeloid leukemia
The recognized prognostic factors in acute myeloid leukemia (AML) have dramatically evolved over the past few years, becoming progressively more complex. Generally, the impact and significance of the older prognostic factors, such as morphology, immunophenotyping, and cytochemistry, which deal with the structure of the malignant cell, are subsiding as new factors dominate, such as cytogenetics and molecular determinants. Despite this evolution, the treatment of AML has not markedly changed except for the increasing use of allogeneic bone marrow transplantation because of an ever-increasing unrelated donor pool and the use of reduced-intensity conditioning for older patients and those with comorbidities. Therefore, currently, the most important implication of the prognostication is in the decision whether to send patients to allogeneic transplant in the first complete remission (CR).
Patient-Related Prognostic Factors
Age
Age is a major prognostic factor in most of the hematological malignancies, including AML. Older patients have a poorer performance status and more comorbidities. Their leukemia is more frequently secondary to an antecedent hematological disorder or is therapy-related and the malignant cells commonly have unfavorable cytogenetics and express the multidrug resistance glycoprotein 1 (MDR1). The Swedish National Acute Leukemia Registry is the largest population-based unselected series that demonstrated that, regardless of management, age is a continuum and also has a strong poor prognostic impact within the group of older patients. Despite the importance of age as a prognostic factor, age alone should not be a barrier to treating patients. Performance status and comorbidities are of paramount consideration.
Performance status
Performance status was historically considered an important prognostic factor in AML. In a recent retrospective analysis from the Medical Research Council (MRC) in Great Britain of more than 1000 patients aged more than 60 years, performance status was found, by multivariate analysis, to be 1 of 5 independent factors that significantly influence prognosis. This finding was subsequently validated by data from another large MRC trial.
Comorbidities
One of the most common explanations for the poor prognosis of older patients is their comorbidities. A retrospective study from the MD Anderson Cancer Center examined the validity of the hematopoietic cell transplantation comorbidity index (HCTCI) in patients with AML aged more than 60 years. This scoring system includes pulmonary, cardiac, hepatic, and renal impairments, as well as psychiatric disturbances, infectious disease, obesity, and a history of a solid tumor. They found that the HCTCI was predictive of an early death rate and overall survival (OS).
Socioeconomic status
A Swedish study, using a cohort of more than 9000 patients with AML in Sweden between 1973 and 2005, observed that white-collar workers had a lower mortality than other socioeconomic status groups ( P = .005). Possible explanations for this observation are biologic differences in tumor characteristics, differences in the health care provider’s attitude toward management, and different frequencies among patients undergoing an allogeneic transplant. In addition, lifestyle factors, such as nutritional status, physical activity, obesity, among others, that are influenced by socioeconomic status may also have an impact on patients’ tolerance.
Race and gender
A retrospective analysis by the Cancer and Leukemia Group B (CALGB) of 2300 whites and 270 African American patients with de novo AML found a lower CR rate ( P = .001) and worse OS ( P = .004) among African American men compared with whites and African American women. A superior prognosis for women was also observed in white populations. The large Swedish study demonstrated a lower relative risk of death ( P = .02) in women compared with men.
Disease-Related Prognostic Factors
White blood cell count
A high white blood cell (WBC) count at diagnosis expresses a high blast count and high tumor burden. Not surprisingly, this variable was historically considered an important poor prognostic factor. It is unknown whether the prognostic effect of a high WBC count is a result of a high tumor burden or is a surrogate for leukemias that have distinct entities with different pathophysiology and clinical characteristics. For example, a high leukocyte count may be associated with a molecular change, like the FLT3-ITD mutation, and the molecular aberration is likely to be responsible for the poor prognosis and not the WBC itself. Furthermore, a precise cutoff defining a high WBC has not been established. Different investigators have preferred different cutoffs of WBC: 20,000/μL, 30,000/μL, 50,000/μL, or 100,000/μL. When the WBC exceeds 100,000/μL, the term hyperleukocytosis is usually used and patients are at an increased risk of developing clinical symptoms of leukostasis. Dutcher and colleagues showed that among patients with AML, those with hyperleukocytosis have a lower CR rate, disease-free survival (DFS), and OS and a high rate of early mortality as opposed to Greenwood and colleagues, who demonstrated that only early mortality was affected by hypoleukocytosis without influence on the other prognostic parameters. In contrast to the importance of a high WBC count at diagnosis, leukopenia at diagnosis does not seem to have any prognostic significance in AML.
Immunophenotyping
Many studies have attempted to find an association between immunophenotyping and prognosis. Some reported on single antigen expression that correlate with poor prognosis and others identified clusters of antigens to be associated to the prognosis. Examples of antigens with a poor prognosis, demonstrated in more than one study include the following: CD7, CD11b, CD14, CD34 and HLA-DR. Li and colleagues demonstrated, in a cohort of more than 800 patients with AML, that the poor-risk antigens correlate not only with poor prognosis but also with unfavorable-risk cytogenetics. This finding confirms that antigens on the leukemic cells may not independently influence the prognosis. They are associated markers for the more important genetic changes.
Other factors, such as thrombocytopenia, organomegaly, and an elevated lactate dehydrogenase, have been proposed in some publications as poor prognostic factors, but these findings are clearly not consistently observed.
Cytogenetics
Cytogenics came into the forefront in AML in the 1970s, when some of the seminal early predictions of the impact on prognosis in AML were reported. Since that time, several refinements have been made because of improved karyotypic resolution. As a result, karyotypic analysis has become indispensable in the management of patients with AML and are now an inherent part of the analysis of data in any large clinical trial in AML. Several classifications have been reported, all following a similar broader outline that, nevertheless, includes important differences. Currently, cytogenetics are considered the single most important factor in the management of patients with AML. An abnormal karyotype is found in about 60% of patients with AML. Several studies in recent years have attempted to divide patients with AML into prognostic groups according to their karyotype. Most of the data from the different studies are consistent and the variations are summarized in Table 1 .
| Original MRC | SWOG/ECOG | CALGB | GIMEMA AML10 | GERMAN AMLCG | HOVON/SAKK | Refined MRC 29 | |
|---|---|---|---|---|---|---|---|
| Favorable | t(15;17) t(8;21) inv(16)/t(16;16) | t(15;17) t(8;21)(lacking del(9q), complex (ie, ≥3 unrel abn) inv(16)/t(16;16)/del(16q) | t(15;17) t(8;21) inv(16)/t(16;16) | t(15;17) t(8;21) inv(16)/t(16;16) | t(15;17) t(8;21) inv(16)/t(16;16) | t(15;17) t(8;21) inv/del(16) & lacking unfav abn | t(15;17) t(8;21) inv(16)/t(16;16) |
| Intermediate | Normal other noncomplex | Normal +6, +8, -Y, del(12p) | Normal other noncomplex | Normal -Y | Normal other noncomplex | Normal other noncomplex | Normal other noncomplex |
| abn(3q) -5/del(5q) -7 complex [≥5 unrel abn] | abn(3q), (9q), (11q), (21q) abn(17p) -5/del(5q) -7/del(7q) t(6;9) t(9:22) complex [≥3 unrel abn] | inv(3)/t(3;3) -7 t(6;9) t(6;11) t(11;19) +8 complex [≥3 unrel abn] | Other | inv(3)/t(3;3) -5/del(5q) -7/del (7q) abn(11q23) del(12p) abn(17p) complex [≥3 unrel abn] | abn(3q) -5/del(5q) -7/del (7q) abn(11q23) t(6;9) t(9;22) complex [≥3 unrel abn] | abn(3q) [excluding t(3;5)] inv(3)/t(3;3) add(5q)/del(5q),/-5, -7/add(7q). t(6;11) t(10;11) t(9;22) -17, abn(17p) with other changes complex [≥3 unrel abn] | |
| Adverse | Excluding those with favorable changes | Excluding those with favorable changes |
Only 3 types of karyotypes are considered as having a favorable outcome after intensive chemotherapy. These karyotypes are the following: t(15;17) in APL, inv(16)/t(16;16), and t(8:21), which are genomic aberrations that are characterized at the molecular stage by the interruption of genes encoding subunits of the core-binding factor (CBF). On the other hand, some types of karyotypes represent adverse prognostic outcomes that are associated with greater resistance to any form of aggressive chemotherapy. These karyotypes include abnormalities of (3q), monosomy 5 or 7, del (5q) or (7q), t(9;22), and a complex karyotype. There are other, less-frequent karyotypes lacking information because of the small number of patients in any group. Patients with a normal karyotype or with aberrations that are not included in the previous groups are considered an intermediate prognostic group. Recently, Grimwade and colleagues summarized the largest cytogenetic cohort of patients with AML containing cytogenetic and clinical information of 5876 younger adults (aged 16–59 years) who were treated in several MRC trials between 1988 and 2009. This study adds information about rare recurring cytogenetic abnormalities and several combinations of aberrations ( Table 2 ). In a multivariate analysis, the poorer outcome observed in this study was in patients with the following: abn(3q) excluding t(3;5)(q25;q34); inv(3)(q21q26)/t(3;3)(q21;q26); add(5q)/del(5q); -5,-7,add(7q)/del(7q); t(6;11)(q27;q23); t(10;11)(p11˜13;q23); other t(11q23), excluding t(9;11)(p21˜22;q23) and t(11;19)(q23;p13); t(9;22)(q34;q11); -17; and abn(17p). Another uncertainty that this study tried to resolve was the definition of a complex karyotype, which, among published studies, ranged between 3 and 5 aberrations. In this study, patients with 4 or more cytogenetic abnormalities, which are not included in the standard (favorable) and poor prognostic ones, were considered as having a complex karyotype with a poor prognosis. It is likely that the unfavorable prognostic group needs to be further subdivided into smaller groups. An important report from the Dutch Hovon group was the first to report on the unfavorable prognosis of patients with the monosomal karyotype, which was defined as 2 or more monosomies or a single monosomy in the presence of structural abnormalities, having an extremely poor prognosis. These data were subsequently confirmed by other groups evaluating a large cohort of patients with AML and correlating with cytogenetic abnormalities.
| Standard | Intermediate | Unfavorable | Uncertain | |
|---|---|---|---|---|
| A. Patient related | ||||
| Age | >50 >65 >75 | |||
| Performance status | Poor | |||
| Comorbidities | Multiple | |||
| Socioeconomic status | White collar | |||
| Race and gender | African American men | |||
| B. Disease related | ||||
| WBC count | More than 20/ 30/ 50/ 100,000/μL | |||
| Immunophenotype | CD7/ 11b/ 14/34, HLA-DR | |||
| Cytogenetics (according the MRC) | t(15;17)(q22;q21), t(8;21)(q22;q22), inv(16)(p13q22)/ t(16;16)(p13;q22) Regardless of additional cytogenetics | Entities not classified as favorable or unfavorable | abn(3q) [excluding t(3;5)(q25;q34)], inv(3)(q21q26)/t(3;3)(q21;q26), add(5q)/del(5q), -5, -7,add(7q)/del(7q), t(6;11)(q27;q23), t(10;11)(p11˜13;q23), other t(11q23) [excluding t(9;11)(p21˜22;q23) and t(11;19)(q23;p13)], t(9;22)(q34;q11), -17, and abn(17p), Complex (≥4 unrelated abnormalities) Excluding cases with favorable karyotype | |
| Molecular diagnosis: | ||||
| CBF AML | T(8;21) + KIT mut | inv(16) + KIT mut | ||
| Normal karyotype | FLT3-ITD | FLT3-TKD | ||
| NPM1 mut FLT3 wt | TET2 | IDH1+IDH2 | ||
| CEBPA dm FLT3 wt | DNMT3A | NRAS+KRAS | ||
| TP53 mut | WT1 | |||
| MDR1 overexposed | MLL-PTD | |||
| Secondary AML (in addition to karyotype) | t-AML in int. cytogenetic group | t-AML in unfavorable group | t-AML in favorable group | |
| C. Response related | ||||
| Day 14–16 marrow | <10% blasts | ≥10% blasts | ||
| In a cellular BM | ||||
| PB blast clearance | Early | |||
| MRD | Negative | Positive | ||
Another type of combination with conflicting reports is the favorable karyotype, such as inv(16), together with additional abnormalities. The data here are limited because of the small numbers of patients in the different studies. The huge cohort of the MRC suggested that additional cytogenetic aberrations do not adversely impact the favorable prognosis of patients with t(15;17), inv(16)/t(16;16), and t(8:21) treated on current intensive protocols. Furthermore, in patients with inv(16), the presence of additional chromosomal changes, such +22, have been reported to predict a better outcome.
The standard or favorable karyotype group accounts for about 20% of young patients with AML (aged <55 years) and about 7% of older patients, although the absolute incidence of patients with the favorable karyotype is not decreased with older age. The CR rate of these patients is high (about 90%), the 5-year OS is about 45% to 65%, and the relapse rate is low. On the other hand, the unfavorable cytogenetic group consists of about 15% to 20% of patients with AML, depending on the age and the different study definitions. The CR rate is less than 50% and the 5-year OS is less than 20%.
The practical implication of assigning patients to prognostic groups relates to the decision regarding which patients should be referred for allogeneic transplant in the first CR. In terms of DFS, the MRC study demonstrated a benefit for transplant mainly in the intermediate group, but the European Organization for Research and Treatment of Cancer – Gruppo Italiano Malattie Ematologiche Maligne dell’Adulto (EORTC-GIMEMA) found benefit only in the unfavorable group. A meta-analysis, which took into consideration these 2 studies together with the Dutch-Belgian Hemato-Oncology Cooperative Group – Swiss Group for Clinical Cancer Research (HOVON-SAKK) and Bordeaux Grenoble Marseille Toulouse Cooperative Group (BGMT) studies, showed a benefit for allogeneic transplant with both intermediate and high-risk groups. The benefit was not only in DFS but also in OS. Despite these conclusions of the meta-analysis, the intermediate risk group, especially the huge subgroup of normal karyotype, still comprises a large heterogeneous group of patients. The need for the refinement of prognostic subgroups has led to the use of molecular markers, which allows for better discrimination of the prognostic impact ( Fig. 1 ).
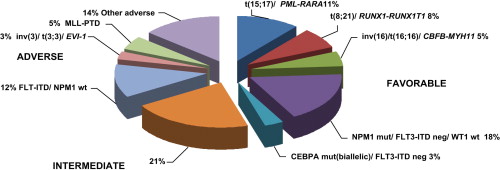
Molecular markers
CBF AMLs
The long-term OS of CBF AMLs is better than other AMLs but is still only about 45%. Thus, the common name of this group as favorable is a misnomer. Defining the patients with the worst prognosis will facilitate sending these patients to allogeneic transplant early and improving their outcome.
KIT mutations
The KIT gene encodes a 145-kD transmembrane glycoprotein, which is a member of the type III receptor tyrosine kinase family. Following ligand binding, the receptor activates downstream signaling pathways involved in proliferation and differentiation. Gain-of-function mutations can cause ligand-independent activation of KIT. In CBF AML, KIT mutations cluster most frequently within exon 17, which encodes the KIT activation loop in the kinase domain, and in exon 8, which encodes a region in the extracellular portion of the KIT receptor that is thought to play a role in receptor dimerization. The incidence of KIT mutation is between 20% to 40% in both t(8;21) and inv(16) in different studies. Some studies examined the prognostic implication of KIT mutation but with different conclusions. In patients with t(8;21), the cumulative incidence of relapse is higher in patients with KIT mutation compared with wild type (WT). Most studies, but not all of them, also showed decreased OS in patients with a mutation. In patients with inv(16), some have reported that OS had an adverse impact among the mutated patients, whereas other studies failed to demonstrate any prognostic impact.
Normal Karyotype
About 40% of AMLs have a normal karyotype and belong to the intermediate prognostic group. Several molecular markers can help to subcategorize this heterogeneous group (see Table 2 ).
Mutations
FLT3 mutations
FLT3 is another member of the type III receptor tyrosine kinase family. It is expressed normally in early progenitors in the bone marrow and has an important part in hematopoiesis. FLT3 is expressed at high levels in 70% to 100% of patients with AML. The most common mutations are the internal tandem duplications (ITD) that occur in about 25% of AMLs. These mutations disrupt the autoinhibitory function of the juxtamembrane domain of the receptor. Patients with these mutations present frequently with a high WBC count, and most of them have normal cytogenetics. Although the CR rate of patients with these mutations is not significantly different from those with the WT, the DFS and OS are reduced. High FLT3 mutant levels tend to worsen the prognosis. Another type of FLT3 mutations, although less frequent, were described in the tyrosine kinase domain. These mutations are also associated with a high WBC count and are most frequent in patients with a normal karyotype, but reports on their prognostic impact is conflicting. Different studies showed positive, negative, or no impact on prognosis. This variability can be related to different combinations of aberrations or to different incidence of biallelic disease.
Nucleophosmin mutations
Nucleophosmin (NPM1) is a nucleolar protein that shuttles between the nucleus and the cytoplasm. It has several functions, including regulation of the transport and import of different particles through the nuclear membrane and interaction with p53 in controlling cell proliferation and apoptosis. NPM1 mutations seem to be founder genetic alterations. These mutations are responsible for the aberrant expression of the nucleolar nucleophosmin in the cytoplasm of the leukemic cells. For this reason, these aberrations can be recognized not only by molecular assays but also by immunohistochemical staining and flow cytometry. The mutations can be found in about one-third of adult AML and about 50% of AMLs with normal karyotype. Several studies demonstrated the favorable effect of NPM1 mutations on prognosis. Some demonstrated this in patients with both FLT3-ITD mutations and FLT3-WT, creating 3 different prognostic groups: NPM1+/FLT3-WT (favorable), NPM1-/FLT3-WT or NPM1+/FLT3-ITD (intermediate), and NPM1-/FLT3-ITD (unfavorable) ( Fig. 2 ). Others confirmed its favorable effect only in patients with FLT3-WT and not in patients with FLT3-ITD. The NPM1 mutations, as founder aberrations, are stable throughout the course of the disease and can be used as markers for MRD assessment and for the detection of early relapse.
CEBPA mutations
CEBPA (CCAAT/enhancer-binding protein alpha) encodes a transcription factor essential for neutrophil development. CEBPA mutations create an imbalance between proliferation and differentiation of hematopoietic progenitors. In patients with a normal karyotype, biallelic mutations have significant advantage in both CR rate and OS. The picture of monoallelic mutations is much less clear. Some reported a survival advantage over WT, and others found both conditions to be the same. No study found monoallelic mutations to be an independent favorable prognostic factor in multivariate analysis. In patients with monoallelic mutations, the presence of FLT3-ITD mutations is still an independent unfavorable prognostic factor (see Fig. 2 ). There are some reports of patients with germ line mutations, part of them with familial AML.
Other mutations
Many other mutations have been described with different importance and varying significance. Mutations of the isocitrate dehydrogenase 1 and 2 genes (IDH1 and IDH2) were described in about 10% to 15% of patients with AML with normal karyotype, but their prognostic impact is not clear. IDH1 mutations may be associated with unfavorable outcomes or do not have any prognostic impact. In IDH2, the variation of descriptions is wider with claims for unfavorable, favorable, and no prognostic impact. RAS mutations (NRAS and KRAS) can be seen in between 12% and 27% of patients with AML and do not have clear outcome implication. There are data to suggest that patients with RAS mutations benefit most from postremission high-dose cytarabine. Wilms tumor gene mutation occurs in about 10% of patients with normal karyotype AML. Here again, its prognostic impact is controversial; although studies of the MRC and CALGB demonstrated association with poorer outcome, a German group did not find any prognostic impact. Partial tandem duplications of the mixed lineage leukemia gene (MLL-PTD) were found in about 10% of normal karyotype AMLs. Most of the studies showed association between these aberrations and unfavorable outcomes but not all of them. TET2 mutations are a common event in a spectrum of myeloid malignancies, including MDS, myeloproliferative disorder, and acute leukemia. A recent study found unfavorable prognosis in patients with AML with TET2 mutations. DNMT3A mutations are associated with poor outcomes. Mutations in TP53 can be seen mainly in patients with complex karyotype and are quite rare in normal karyotype AMLs, and, not surprisingly, they predict for an unfavorable outcome.
Overexpression
Overexpression of some genes has been demonstrated to have significant prognostic impact in patients with normal karyotype AML. Important examples are the Brain and Acute Leukemia Cytoplasmic gene (BAALC), Meningioma1 gene, and Ectopic Virus Integration 1 gene. The interpretation of such quantitative tests is complex and it holds within it questions of standardization and establishment of reference values.
Multidrug resistance
Overexpression of some genes is responsible for high levels of resistance proteins that disturb the influence of chemotherapeutic agents on the malignant cells. The most important one is the MDR1. This gene encodes for a P-glycoprotein, which is a drug efflux protein that decreases anthracyclines accumulation in the cells resulting in the development of anthracyclines-resistant cells. MDR1 expression increases with age and has a significant independent adverse influence on both CR rate and OS. This influence is also relevant when looking on a subgroup of patients with AML with intermediate-risk cytogenetics. Overexpression of other drug-resistance genes, such as as MRP1 and lung resistance protein gene, has less significant prognostic influence on patients with AML.
Secondary AML
Secondary AML is a broad definition that includes AMLs secondary to an antecedent stem cell disorder, like MDS or myeloproliferative neoplasm, or secondary to past chemotherapeutic/radiotherapeutic treatment (t-AML). These leukemias are thought to have a worse prognosis. The main reason is that most of the secondary AMLs have cytogenetic abnormalities associated with a poor outcome. Whether the outcome of secondary AML is a result of the unfavorable cytogenetics only or is also independently related to the history of the disease has been a controversial issue. The German AML Cooperative Group (AMLCG) compared karyotype and survival between patients with t-AML and those with primary AML. They found that in the t-AML, the percentage of patients with unfavorable karyotype was higher than in the de novo patients. The median OS was significantly shorter in the t-AML group. Favorable and unfavorable cytogenetics had a significant survival impact in the t-AML group. In an update of this study, it was reported that in the favorable and unfavorable group, the t-AMLs do worse than their parallels in the de novo group. In contrast, in the intermediate karyotype group, there was no significant difference between the groups. Another study reported that CBF t-AMLs have similar results and the same characteristics as de novo patients, but more recent studies from the MD Anderson Cancer Center found that the CBF t-AML have worse outcomes than the de novo ones.
Regarding AML secondary to MDS, the German AMLCG demonstrated that dysplasia by itself has no independent prognostic impact under the conditions of intensive induction therapy but is related in many cases to an unfavorable karyotype.
Response-Related Prognostic Factors
Early response
A different approach for the prediction of an individual outcome is by evaluation of early response parameters. The amount of residual leukemic blasts in bone marrow on the sixteenth day from the beginning of the induction therapy was demonstrated, in the German AMLCG, to predict for a long-term outcome, even in patients who achieved CR. Others showed that the number of blasts (less or more than 5%) on day 14 can predict CR but not long-term outcomes. There is also evidence for an association between the high number of plasma cells in day 14 marrow and residual leukemia. An Italian group attempted to substitute early bone marrow examination by following the clearance of the leukemic blasts from the peripheral blood using daily flow cytometry. They found correlation between the clearance rate and the day 14 marrow.
Cytogenetic CR
Achievement of CR is a requirement, although not sufficient, for cure. The CALGB demonstrated that patients with abnormal karyotype at diagnosis, in whom the bone marrow was converted to normal karyotype at first CR, have significantly better outcomes.
MRD
MRD is a developing and promising risk-assessment system. The model of MRD in AML came from APL, in which real-time quantitative polymerase chain reaction (RQ-PCR) for PML-RARA transcript level, after consolidation, became the follow-up standard of care. Basically, there are 2 main methods of MRD assessment based on PCR amplification of molecular abnormalities and flow cytometric detection of abnormal immunophenotypes. RQ-PCR is now considered the preferred PCR method because it is less prone to contamination, allows the kinetics of disease response and relapse to be defined, and enables poor quality samples that could have given rise to false negative results according to older PCR assays to be identified based on the level of endogenous control gene transcripts (eg, ABL ). The PCR method is based on amplification of fusion transcripts as AML1-ETO, CBFbeta/MYH11 and MLL-AF9 with the limitation that only about one-third of patients with AML have these transcripts. Patients with persistent high levels of fusion transcripts at the end of consolidation or reappearance of transcripts after molecular remission are prone to early relapse. Other options are based on the detection of overexpressed genes, like WT1, or mutated genes, like FLT3-ITD and NPM1. The European Leukemia study demonstrated that greater WT1 transcript reduction after induction can predict the relapse risk, although the background of normal bone marrow cells may limit the reliability of this assay. NPM1 MRD level was shown, by multivariate analysis, to be an important prognostic factor but FLT3-ITD is more controversial as an MRD marker because of the observation that this marker may not be stable during the course of disease. The other method of MRD detection uses flow cytometry for identifying the aberrant expression pattern of cellular markers on the leukemic cells. In about 75% of patients with AML there is abnormal immunophenotype. Because the immunophenotype can distinguish the leukemic cells from the normal bone marrow cells, the sensitivity of the test improves, in the range of between 1 leukemic cell among 1000 to 10,000 normal cells. An Italian study demonstrated that a bone marrow with more than 3.5 X 10 −4 residual leukemic cells after consolidation is considered MRD positive and carries a poor prognosis. The limitations of this method are the option of immunophenotypic changes that may occur during the course of the disease and that the results depend on the investigator interpretation.
Scores
Because of the large number of prognostic factors in AML, many groups tried to compose prognostic scores that take into account some factors together. For example, a Korean group developed a model based on MDR status and cytogenetics. An Italian group developed a prognostic scoring for cytogenetically normal patients that rely on age of more than 50 years, secondary AML, and a WBC greater than 20,000/ml. A European group recently published an integrative prognostic risk score for normal karyotype AMLs based on age; WBC; and several molecular markers, like NPM1, FLT3-ITD, BAALC, WT1, and others. The main problem of these and of many other models is that they were not prospectively validated in large cohorts.
Molecular markers
CBF AMLs
The long-term OS of CBF AMLs is better than other AMLs but is still only about 45%. Thus, the common name of this group as favorable is a misnomer. Defining the patients with the worst prognosis will facilitate sending these patients to allogeneic transplant early and improving their outcome.
KIT mutations
The KIT gene encodes a 145-kD transmembrane glycoprotein, which is a member of the type III receptor tyrosine kinase family. Following ligand binding, the receptor activates downstream signaling pathways involved in proliferation and differentiation. Gain-of-function mutations can cause ligand-independent activation of KIT. In CBF AML, KIT mutations cluster most frequently within exon 17, which encodes the KIT activation loop in the kinase domain, and in exon 8, which encodes a region in the extracellular portion of the KIT receptor that is thought to play a role in receptor dimerization. The incidence of KIT mutation is between 20% to 40% in both t(8;21) and inv(16) in different studies. Some studies examined the prognostic implication of KIT mutation but with different conclusions. In patients with t(8;21), the cumulative incidence of relapse is higher in patients with KIT mutation compared with wild type (WT). Most studies, but not all of them, also showed decreased OS in patients with a mutation. In patients with inv(16), some have reported that OS had an adverse impact among the mutated patients, whereas other studies failed to demonstrate any prognostic impact.
Normal Karyotype
About 40% of AMLs have a normal karyotype and belong to the intermediate prognostic group. Several molecular markers can help to subcategorize this heterogeneous group (see Table 2 ).
Mutations
FLT3 mutations
FLT3 is another member of the type III receptor tyrosine kinase family. It is expressed normally in early progenitors in the bone marrow and has an important part in hematopoiesis. FLT3 is expressed at high levels in 70% to 100% of patients with AML. The most common mutations are the internal tandem duplications (ITD) that occur in about 25% of AMLs. These mutations disrupt the autoinhibitory function of the juxtamembrane domain of the receptor. Patients with these mutations present frequently with a high WBC count, and most of them have normal cytogenetics. Although the CR rate of patients with these mutations is not significantly different from those with the WT, the DFS and OS are reduced. High FLT3 mutant levels tend to worsen the prognosis. Another type of FLT3 mutations, although less frequent, were described in the tyrosine kinase domain. These mutations are also associated with a high WBC count and are most frequent in patients with a normal karyotype, but reports on their prognostic impact is conflicting. Different studies showed positive, negative, or no impact on prognosis. This variability can be related to different combinations of aberrations or to different incidence of biallelic disease.
Nucleophosmin mutations
Nucleophosmin (NPM1) is a nucleolar protein that shuttles between the nucleus and the cytoplasm. It has several functions, including regulation of the transport and import of different particles through the nuclear membrane and interaction with p53 in controlling cell proliferation and apoptosis. NPM1 mutations seem to be founder genetic alterations. These mutations are responsible for the aberrant expression of the nucleolar nucleophosmin in the cytoplasm of the leukemic cells. For this reason, these aberrations can be recognized not only by molecular assays but also by immunohistochemical staining and flow cytometry. The mutations can be found in about one-third of adult AML and about 50% of AMLs with normal karyotype. Several studies demonstrated the favorable effect of NPM1 mutations on prognosis. Some demonstrated this in patients with both FLT3-ITD mutations and FLT3-WT, creating 3 different prognostic groups: NPM1+/FLT3-WT (favorable), NPM1-/FLT3-WT or NPM1+/FLT3-ITD (intermediate), and NPM1-/FLT3-ITD (unfavorable) ( Fig. 2 ). Others confirmed its favorable effect only in patients with FLT3-WT and not in patients with FLT3-ITD. The NPM1 mutations, as founder aberrations, are stable throughout the course of the disease and can be used as markers for MRD assessment and for the detection of early relapse.







