Advances in the understanding of the molecular pathogenesis of acute myeloid leukemia have led to the identification of diagnostic and prognostic markers, resulting in further refinement of karyotype-based risk classification. This article presents strategies for induction therapy of younger adults and approaches to postremission therapy based on prognostic features. Although some older adults may benefit from intensive chemotherapy and reduced-intensity hematopoietic stem cell transplantation, development of less intensive therapies for older patients who are not candidates for these modalities is an area of active investigation. Novel molecularly targeted agents and other investigational chemotherapeutic drugs now entering late-stage development are also discussed.
Acute myeloid leukemia (AML) represents a group of heterogeneous neoplasms with acquired genetic abnormalities that result in impaired differentiation and increased proliferation of hematopoietic cells. AML is a rare disease with an incidence rate in the United States of approximately 3.5 per 100,000 persons. Among individuals aged 65 years and older, the incidence increases dramatically to more than 16 per 100,000 persons. Despite modest improvements in the outcome of younger patients over the past decade, overall treatment results remain disappointing, particularly for older individuals. Data from the Surveillance, Epidemiology, and End Results program of the National Cancer Institute indicate that the 5-year survival rate for patients less than age 65 years, diagnosed from 2001 to 2007, is approximately 40%. In contrast, the 5-year survival rate for patients 65 years and older is only 5%. New understanding of the molecular pathogenesis of this disease has led to the identification of diagnostic and prognostic markers and to the refinement of karyotype-based risk classification, discussed elsewhere in this issue. These insights are now beginning to guide therapeutic strategies and provide novel targets for drug discovery.
Induction therapy for younger adults
Elimination of morphologic evidence of leukemic blasts and restoration of normal hematopiesis are the goals of induction therapy. For the past two decades, the combination of anthracycline or anthracycline-like drugs with cytarabine has remained the standard of care for patients aged 60 years or less. With 3 days of daunorubicin (at least 60 mg/m 2 /d), idarubicin (10–12 mg/m 2 /d), or mitoxantrone (10–12 mg/m 2 /d), and 7 days of cytarabine (100–200 mg/m 2 /d continuous intravenous infusion), complete remission (CR) is obtained in 60% to 80% of patients less than 60 years of age. Most centers use one of the previously mentioned combinations, sometimes with the addition of etoposide, although this has been shown to have a limited benefit in patients less than age 55 years. AML is still considered one of the few true oncologic emergencies; prompt initiation of therapy seems to be crucial. In one retrospective study, treatment delays beyond 5 days from diagnosis resulted in a decreased response rate and overall survival (OS) in patients less than 55 years of age.
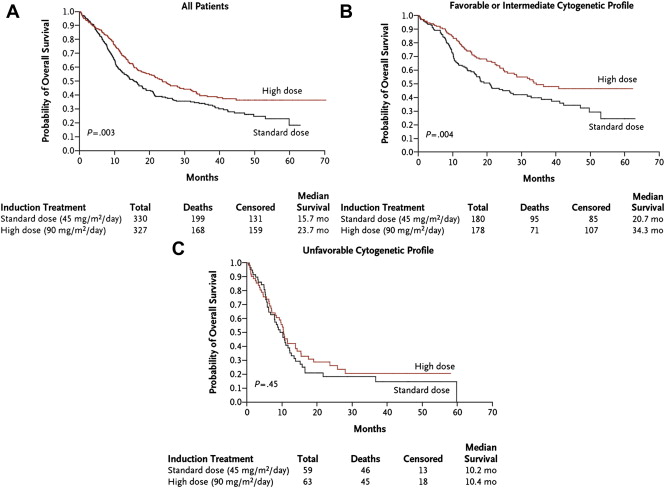
To improve response rates and OS, investigators have explored several modifications to standard induction approaches. Studies comparing idarubicin and mitoxantrone with daunorubicin at doses of 45 to 60 mg/m 2 have failed to show a clear survival benefit. More recently, dose intensification of daunorubicin (90 vs 45 mg/m 2 ) was reported to result in a higher CR rate and OS in patients younger than 60 years of age, particularly those with favorable- or intermediate-risk disease ( Fig 1 ). The Acute Leukemia French Association (ALFA) compared high-dose daunorubicin (80 mg/m 2 for 3 days) with idarubicin (12 mg/m 2 for 3 or 4 days) combined with intermediate-dose cytarabine for induction in patients 50 to 70 years of age. Although the CR rate was significantly higher using the 3-day schedule of idarubicin, no differences in event-free survival (EFS) or OS were observed among the three groups. Similarly, the Japan Adult Leukemia Study Group compared high-dose daunorubicin (50 mg/m 2 for 5 days) with idarubicin (12 mg/m 2 for 3 days) in combination with standard-dose cytarabine and found no differences in CR or OS rates.
Given that high-dose cytarabine (HiDAC) can overcome resistance to standard doses of cytarabine, its use has been evaluated in induction with cumulative doses of 18 to 24 g/m 2 . This approach did not improve OS and generally was associated with increased toxicity. The Dutch-Belgian Cooperative Trial Group for Hemato-Oncology (HOVON) and the Swiss Group for Clinical Cancer research (SAKK) recently compared intermediate-dose cytarabine (200 mg/m 2 for 7 days by continuous infusion in cycle 1 and 1000 mg/m 2 over 3 hours twice daily for 6 days in cycle 2) with HiDAC (1000 mg/m 2 twice daily for 5 days in cycle 1 and 2000 mg/m 2 twice daily over 6 hours for 4 days in cycle 2) during induction. No differences in remission rate, relapse rate, EFS, or OS were seen; however, the high-dose regimen was associated with increased toxicity. A subgroup analysis of this trial and data from the Southwest Oncology Group (SWOG) suggest that treatment with higher doses of cytarabine may confer a survival benefit for patients with a highly unfavorable monosomal karyotype. Nevertheless, these results provide evidence for a plateau in the dose-response for cytarabine and argue against the use of HiDAC during induction outside the setting of a clinical trial.
Similarly, the use of additional cytotoxic agents (etoposide, fludarabine, topotecan, and thioguanine) during induction has not shown a clear advantage. Neither cyclosporine nor valspodar (PSC-833), which act as modulators of multidrug resistance by inhibiting P-glycoprotein–mediated cellular export of anthracyclines, improved results compared with standard therapy. During induction and consolidation, myeloid growth factors may be given to reduce the duration of neutropenia. Although the safety of these agents in AML has been clearly established in multiple trials, benefits are limited. In general, the use of myeloid growth factors is recommended primarily in older individuals, where the risk of periinduction mortality because of infectious complications is greatest; however, their use should also be considered in younger patients with severe infection after induction. The use of granulocyte colony-stimulating factor (G-CSF) and granulocyte macrophage (GM)-CSF as sensitizing agents has also been evaluated because of the potential to act as priming agents by moving leukemia cells into a phase of the cell cycle more susceptible to cytotoxic chemotherapy. Conflicting results have been reported, and no clear benefit has been established. The HOVAN/SAKK group found that priming with G-CSF resulted in a significant improvement in disease-free survival (DFS) and superior OS in intermediate-risk disease. The CR rate, however, was unaffected. The ALFA group found that priming with GM-CSF improved CR and EFS rates but had no effect on OS. In contrast, a study by the AML Cooperative Group found that G-CSF priming had no impact on relapse-free survival or OS.
Two large studies tested the addition of antibody-directed chemotherapy with gemtuzumab ozogamicin (GO) to standard induction chemotherapy. GO consists of a recombinant humanized anti-CD33 monoclonal antibody conjugated to calicheamicin, a potent antitumor antibiotic. Within the acidic environment of lysosomes after internalization, calicheamicin dissociates from the antibody and migrates to the nucleus, where it causes double-stranded DNA breaks. As a single agent, GO produced remissions in 26% of older patients with AML in first relapse. When combined with standard chemotherapy, dose reductions of GO were necessary to avoid hepatic toxicity. A randomized trial conducted by SWOG compared induction with daunorubicin, cytarabine, and GO with daunorubicin and cytarabine alone. The trial was stopped early after an interim analysis showed an increase in periinduction mortality in the GO arm (5.8% vs 0.8%). The early death rate in the control arm, however, was significantly lower than expected. Additionally, no differences in CR rate (66% vs 69%), relapse-free survival, DFS, or OS were seen between the two groups. In a larger study conducted by the United Kingdom National Cancer Research Institute (NCRI) (formerly the Medical Research Council), patients were randomized to receive one of three chemotherapy regimens (daunorubicin and cytarabine; cytarabine, daunorubicin, and etoposide; or fludarabine, cytarabine, G-CSF, and idarubicin), with or without a single dose of GO. Although there was no difference in response or survival among the arms, in prespecified risk groups, there was a significant survival benefit in patients with favorable cytogenetics. Moreover, an internally validated prognostic index (based on age, presenting leukocyte count, performance status, and presence of secondary AML) identified 70% of patients with intermediate-risk cytogenetics who benefited with a 10% improvement in survival at 5 years. Although marketing authorization of GO was withdrawn in the United States because of concerns about safety and lack of efficacy in the pivotal SWOG trial, the NCRI study suggests that it may have a role in induction therapy for selected patients.
Postremission therapy for younger patients
Several postremission treatment strategies have been studied to eradicate minimal residual disease in patients with AML who achieve a CR but remain at risk for relapse. These include intensive conventional chemotherapy, autologous or allogeneic hematopoietic cell transplantation (HCT), and prolonged low-dose maintenance therapy. The choice of postremission strategy for an individual patient depends on cytogenetic and molecular risk factors, availability of a suitable allogeneic stem cell donor, and predicted treatment-related mortality (TRM).
Intensive Chemotherapy
A pivotal study by the Cancer and Leukemia Group B (CALGB) examined three dose levels of cytarabine given for four cycles as postremission therapy. This study clearly demonstrated that outcomes after HiDAC (3 g/m 2 every 12 hours on days 1, 3, and 5) were superior to intermediate-dose (400 mg/m 2 continuous infusion for 5 days) or standard-dose cytarabine (100 mg/m 2 continuous infusion for 5 days) in patients less than age 60 years. A subgroup analysis demonstrated that this benefit was most pronounced in patients with core-binding factor (CBF; ie, t[8;21][q22;q22], inv[16][p13.1;q22], t[16;16][p13.1;q22]) AML and, to a lesser extent, in patients with a normal karyotype. Although the benefit of HiDAC consolidation has been demonstrated in younger patients, the optimal dose, schedule, and number of cycles has not been established definitively. Retrospective studies by the CALGB suggested that three cycles or more of HiDAC was superior to only one cycle. The NCRI, however, reported a similar OS rate with only one course of HiDAC in consolidation for patients with favorable-risk cytogenetics. Moreover, results of a US Intergroup study that included only one cycle of postremission HiDAC were similar to outcomes in other studies where three or four cycles of HiDAC were used. More recently, the NCRI reported that outcomes after consolidation with two or three courses of cytarabine at doses of 1.5 or 3 g/m 2 for six doses were equivalent. These results, however, may be confounded by the use of variable induction regimens and the administration of GO to some patients during consolidation.
For younger patients with CBF leukemia, three or four cycles of HiDAC consolidation remains appropriate postremission therapy, resulting in an OS rate of 60% to 75%. A survival benefit has not been demonstrated for patients with CBF leukemia with autologous or allogeneic HCT in first CR (CR1). Despite this, there may be a subset of CBF leukemia patients with additional risk factors for relapse, including KIT mutations and elevated WBC in t(8;21), who may benefit from allogeneic HCT in CR1 if expected TRM is sufficiently low. HiDAC consolidation may also be appropriate for at least a subgroup younger patients with normal cytogenetics and mutated nucleophosmin gene ( NPM1 ) without the fms-related tyrosine kinase 3 gene ( FLT3 ) internal tandem duplication (ITD) mutation. In a retrospective study, the German-Austrian AML Study Group reported a similar outcome for patients with or without a suitable related stem cell donor who had a normal karyotype and were NPM1 + and FLT3 -ITD − . More recent data, however, suggest that the favorable prognostic impact of the NPM1 mutation occurs primarily in patients without mutations in isocitrate dehydrogenase 1 ( IDH1 ) and IDH2 genes. Even further, additional data suggest that the location of the mutation in IDH2 influences patient outcome. When there are concomitant mutations in NPM1 and IDH2 at R140, the prognosis is favorable, whereas an IDH2 mutation at R172 is associated with a poor outcome. The therapeutic implications of these recently described molecular abnormalities are unclear. Additional studies are required to determine which patients with NPM1 mutations may benefit from allogeneic HCT in CR1 and which will have superior outcomes with postremission chemotherapy alone. Intensive consolidation may also be appropriate for patients with a normal karyotype who have a mutated CCAAT/enhancer binding protein α ( CEBPA ) gene. It has recently been shown that the improved prognosis in CEBPA + patients is only seen with the double mutation. A single CEBPA mutation, even without the presence of a FLT3- ITD mutation, does not seem to confer the same favorable long-term outcomes.
Allogeneic Hematopoietic Stem Cell Transplantation
Allogeneic HCT is highly effective therapy for AML because of the immunologically mediated graft-versus-leukemia effect and the intensive antileukemic effects of conditioning regimens. With this in mind, it is not surprising that the relapse rate for patients with AML in CR1 is lowest with allogeneic HCT. However, this strong antileukemic effect is balanced by a higher TRM rate compared with chemotherapy alone. In patients with AML in CR1, multiple prospective trials have failed to show an improvement in OS with allogeneic HCT. In patients with intermediate- and high-risk AML, however, a clear OS advantage was shown in several metaanalyses that prospectively assigned allogeneic HCT versus consolidation chemotherapy or autologous HCT in an intent-to-treat donor versus no-donor fashion.
Patients with AML and normal cytogenetics lacking the specific good-risk genetic mutations discussed previously are often treated with standard HiDAC consolidation; however, the risk of relapse remains high in this patient population. The OS benefit of allogeneic HCT has been shown in AML with normal cytogenetics, particularly in the absence of a mutated NMP1 without FLT3-ITD or mutated CEBPA. For patients who have normal cytogenetics and FLT3-ITD mutation, an allogeneic HCT transplant in CR1 should be considered. More recently, mutations in TET2 were described in patients with AML with a frequency of approximately 12%. TET2 abnormalities were associated with decreased OS compared with TET2 wild-type patients. The therapeutic implications of this finding, however, are still unknown.
Outcomes for patients with unfavorable cytogenetics after standard chemotherapy consolidation or autologous HCT are poor. The worst prognosis has been reported for patients with one or more autosomal monosomies. Breems and colleagues reported a 4-year OS of 12% with a single monosomy and a 4-year OS of 3% for patients with two or more monosomies. In contrast, patients with non-CBF cytogenetic abnormalities had a 4-year OS of 27%. Based on results from individual trials and meta-analyses, allogeneic HCT in CR1 with a matched, related donor is recommended for patients with poor-risk cytogenetics.
The use of a matched unrelated donor (MUD) for allogeneic HCT in CR1 is not as clearly defined, because most published trials are retrospective comparisons. The only study to address this issue prospectively is the German AML 01/99 trial. Patients with high-risk disease, defined as having unfavorable cytogenetics or residual disease on day 15 postinduction, were treated with allogeneic HCT from a matched sibling if available, a MUD HCT if such a donor could be identified, or autologous HCT otherwise. Survival at 4 years was 68% for patients with an HLA-matched sibling donor, 56% for patients with a MUD, and 23% for patients allocated to autologous HCT. A retrospective analysis reported by the Fred Hutchinson Cancer Research Center showed that 5-year survival rates were equivalent between matched sibling and unrelated allografts in CR1 at 63% and 61%, respectively, for all risk groups. These data suggest that indications for HCT in AML using a matched related or 10 of 10 MUD should be similar. Further large-scale studies are still required to confirm this finding. The Center for International Blood and Marrow Transplant Research, however, demonstrated only a 30% 5-year survival for poor-risk patients with AML in CR1 after transplant with a MUD. Nevertheless, given the high risk for relapse for patients with unfavorable cytogenetics, allogeneic HCT from either a matched sibling or MUD should be pursued in CR1.
There are emerging data that HCT using alternative sources of hematopoietic stem cells may be useful in younger patients with high-risk AML. Although there are limited data regarding umbilical cord blood transplantation for AML in CR1, this modality may represent a reasonable option for patients without a matched related or unrelated donor. Initially, the widespread use of this approach was restricted by stem cell dose requirements. Double cord blood transplantation, however, seems to lessen the risk of graft rejection and improve early survival, making this approach more broadly applicable to adult patients. Investigators from the University of Minnesota and the Fred Hutchinson Cancer Research Center showed that leukemia-free survival for HCT recipients from double umbilical cord blood, matched related donors, MUD, and mismatched unrelated donors was similar ( Fig. 2 ). The use of a genetically haploidentical donor for HCT is another possible approach for poor-risk patients without a suitable matched sibling or unrelated donor. The European Blood and Marrow Transplant Group reported results after haploidentical HCT in high-risk patients that are similar to those after MUD transplantation.
Autologous Hematopoietic Stem Cell Transplantation
Autologous HCT after intensive chemotherapy is considered a reasonable option for postremission therapy in favorable- and intermediate-risk cytogenetic groups. In patients with poor-risk cytogenetics, however, its use cannot be recommended. Although a benefit in DFS has been reported in some randomized studies, OS was not improved. This is related in part to the higher TRM in studies where bone marrow was used as the source of hematopoietic stem cells. The data are further confounded by the intensity of prior therapy and patient selection. For example, in a recent Eastern Cooperative Oncology Group (ECOG) study, patients with favorable- and intermediate-risk disease received postremission therapy consisting of two courses of HiDAC followed by a course of GO and autologous HCT or autologous HCT alone. Only 49% of patients received the planned therapy, with disease progression as the most common reason for patients not proceeding to HCT. An intention-to-treat analysis demonstrated 4-year DFS and OS of approximately 35% and 41%, respectively, with no benefit from GO. Nevertheless, in favorable-risk AML, patients receiving induction that included high-dose daunorubicin who underwent autologous HCT, 4-year DFS and OS rates were 60% and 80%, respectively. For intermediate-risk patients, 4-year DFS and OS were 40% and 49%, respectively ( Fig. 3 ). These data suggest that autologous HCT may play a role in postremission therapy for a selected subgroup of patients with AML.
Maintenance Therapy
Although several trials have demonstrated an increase in DFS with maintenance therapy after intensive induction, an OS benefit has not been shown. Therefore, for younger patients, maintenance chemotherapy cannot replace more intensive postremission strategies. Immunotherapeutic approaches for maintenance therapy have also been proposed. Although several randomized studies failed to show an advantage for interleukin-2 as maintenance, a recent study showed that interleukin-2 and histamine dichloride given after the completion of consolidation therapy conferred a leukemia-free survival advantage compared with no maintenance therapy. Novel vaccination strategies are also under investigation for maintenance therapy. Wilms’ tumor antigen ( WT1 ) and proteinase 3 (PR1) peptide vaccines have elicited immune responses and produced remissions in patients with AML. Cellular therapies, such as autologous dendritic cell vaccination targeting WT1, also hold promise for postremission immunotherapy. Nevertheless, maintenance is currently not recommended outside of a clinical trial for non-APL AML.
Postremission therapy for younger patients
Several postremission treatment strategies have been studied to eradicate minimal residual disease in patients with AML who achieve a CR but remain at risk for relapse. These include intensive conventional chemotherapy, autologous or allogeneic hematopoietic cell transplantation (HCT), and prolonged low-dose maintenance therapy. The choice of postremission strategy for an individual patient depends on cytogenetic and molecular risk factors, availability of a suitable allogeneic stem cell donor, and predicted treatment-related mortality (TRM).
Intensive Chemotherapy
A pivotal study by the Cancer and Leukemia Group B (CALGB) examined three dose levels of cytarabine given for four cycles as postremission therapy. This study clearly demonstrated that outcomes after HiDAC (3 g/m 2 every 12 hours on days 1, 3, and 5) were superior to intermediate-dose (400 mg/m 2 continuous infusion for 5 days) or standard-dose cytarabine (100 mg/m 2 continuous infusion for 5 days) in patients less than age 60 years. A subgroup analysis demonstrated that this benefit was most pronounced in patients with core-binding factor (CBF; ie, t[8;21][q22;q22], inv[16][p13.1;q22], t[16;16][p13.1;q22]) AML and, to a lesser extent, in patients with a normal karyotype. Although the benefit of HiDAC consolidation has been demonstrated in younger patients, the optimal dose, schedule, and number of cycles has not been established definitively. Retrospective studies by the CALGB suggested that three cycles or more of HiDAC was superior to only one cycle. The NCRI, however, reported a similar OS rate with only one course of HiDAC in consolidation for patients with favorable-risk cytogenetics. Moreover, results of a US Intergroup study that included only one cycle of postremission HiDAC were similar to outcomes in other studies where three or four cycles of HiDAC were used. More recently, the NCRI reported that outcomes after consolidation with two or three courses of cytarabine at doses of 1.5 or 3 g/m 2 for six doses were equivalent. These results, however, may be confounded by the use of variable induction regimens and the administration of GO to some patients during consolidation.
For younger patients with CBF leukemia, three or four cycles of HiDAC consolidation remains appropriate postremission therapy, resulting in an OS rate of 60% to 75%. A survival benefit has not been demonstrated for patients with CBF leukemia with autologous or allogeneic HCT in first CR (CR1). Despite this, there may be a subset of CBF leukemia patients with additional risk factors for relapse, including KIT mutations and elevated WBC in t(8;21), who may benefit from allogeneic HCT in CR1 if expected TRM is sufficiently low. HiDAC consolidation may also be appropriate for at least a subgroup younger patients with normal cytogenetics and mutated nucleophosmin gene ( NPM1 ) without the fms-related tyrosine kinase 3 gene ( FLT3 ) internal tandem duplication (ITD) mutation. In a retrospective study, the German-Austrian AML Study Group reported a similar outcome for patients with or without a suitable related stem cell donor who had a normal karyotype and were NPM1 + and FLT3 -ITD − . More recent data, however, suggest that the favorable prognostic impact of the NPM1 mutation occurs primarily in patients without mutations in isocitrate dehydrogenase 1 ( IDH1 ) and IDH2 genes. Even further, additional data suggest that the location of the mutation in IDH2 influences patient outcome. When there are concomitant mutations in NPM1 and IDH2 at R140, the prognosis is favorable, whereas an IDH2 mutation at R172 is associated with a poor outcome. The therapeutic implications of these recently described molecular abnormalities are unclear. Additional studies are required to determine which patients with NPM1 mutations may benefit from allogeneic HCT in CR1 and which will have superior outcomes with postremission chemotherapy alone. Intensive consolidation may also be appropriate for patients with a normal karyotype who have a mutated CCAAT/enhancer binding protein α ( CEBPA ) gene. It has recently been shown that the improved prognosis in CEBPA + patients is only seen with the double mutation. A single CEBPA mutation, even without the presence of a FLT3- ITD mutation, does not seem to confer the same favorable long-term outcomes.
Allogeneic Hematopoietic Stem Cell Transplantation
Allogeneic HCT is highly effective therapy for AML because of the immunologically mediated graft-versus-leukemia effect and the intensive antileukemic effects of conditioning regimens. With this in mind, it is not surprising that the relapse rate for patients with AML in CR1 is lowest with allogeneic HCT. However, this strong antileukemic effect is balanced by a higher TRM rate compared with chemotherapy alone. In patients with AML in CR1, multiple prospective trials have failed to show an improvement in OS with allogeneic HCT. In patients with intermediate- and high-risk AML, however, a clear OS advantage was shown in several metaanalyses that prospectively assigned allogeneic HCT versus consolidation chemotherapy or autologous HCT in an intent-to-treat donor versus no-donor fashion.
Patients with AML and normal cytogenetics lacking the specific good-risk genetic mutations discussed previously are often treated with standard HiDAC consolidation; however, the risk of relapse remains high in this patient population. The OS benefit of allogeneic HCT has been shown in AML with normal cytogenetics, particularly in the absence of a mutated NMP1 without FLT3-ITD or mutated CEBPA. For patients who have normal cytogenetics and FLT3-ITD mutation, an allogeneic HCT transplant in CR1 should be considered. More recently, mutations in TET2 were described in patients with AML with a frequency of approximately 12%. TET2 abnormalities were associated with decreased OS compared with TET2 wild-type patients. The therapeutic implications of this finding, however, are still unknown.
Outcomes for patients with unfavorable cytogenetics after standard chemotherapy consolidation or autologous HCT are poor. The worst prognosis has been reported for patients with one or more autosomal monosomies. Breems and colleagues reported a 4-year OS of 12% with a single monosomy and a 4-year OS of 3% for patients with two or more monosomies. In contrast, patients with non-CBF cytogenetic abnormalities had a 4-year OS of 27%. Based on results from individual trials and meta-analyses, allogeneic HCT in CR1 with a matched, related donor is recommended for patients with poor-risk cytogenetics.
The use of a matched unrelated donor (MUD) for allogeneic HCT in CR1 is not as clearly defined, because most published trials are retrospective comparisons. The only study to address this issue prospectively is the German AML 01/99 trial. Patients with high-risk disease, defined as having unfavorable cytogenetics or residual disease on day 15 postinduction, were treated with allogeneic HCT from a matched sibling if available, a MUD HCT if such a donor could be identified, or autologous HCT otherwise. Survival at 4 years was 68% for patients with an HLA-matched sibling donor, 56% for patients with a MUD, and 23% for patients allocated to autologous HCT. A retrospective analysis reported by the Fred Hutchinson Cancer Research Center showed that 5-year survival rates were equivalent between matched sibling and unrelated allografts in CR1 at 63% and 61%, respectively, for all risk groups. These data suggest that indications for HCT in AML using a matched related or 10 of 10 MUD should be similar. Further large-scale studies are still required to confirm this finding. The Center for International Blood and Marrow Transplant Research, however, demonstrated only a 30% 5-year survival for poor-risk patients with AML in CR1 after transplant with a MUD. Nevertheless, given the high risk for relapse for patients with unfavorable cytogenetics, allogeneic HCT from either a matched sibling or MUD should be pursued in CR1.
There are emerging data that HCT using alternative sources of hematopoietic stem cells may be useful in younger patients with high-risk AML. Although there are limited data regarding umbilical cord blood transplantation for AML in CR1, this modality may represent a reasonable option for patients without a matched related or unrelated donor. Initially, the widespread use of this approach was restricted by stem cell dose requirements. Double cord blood transplantation, however, seems to lessen the risk of graft rejection and improve early survival, making this approach more broadly applicable to adult patients. Investigators from the University of Minnesota and the Fred Hutchinson Cancer Research Center showed that leukemia-free survival for HCT recipients from double umbilical cord blood, matched related donors, MUD, and mismatched unrelated donors was similar ( Fig. 2 ). The use of a genetically haploidentical donor for HCT is another possible approach for poor-risk patients without a suitable matched sibling or unrelated donor. The European Blood and Marrow Transplant Group reported results after haploidentical HCT in high-risk patients that are similar to those after MUD transplantation.
Autologous Hematopoietic Stem Cell Transplantation
Autologous HCT after intensive chemotherapy is considered a reasonable option for postremission therapy in favorable- and intermediate-risk cytogenetic groups. In patients with poor-risk cytogenetics, however, its use cannot be recommended. Although a benefit in DFS has been reported in some randomized studies, OS was not improved. This is related in part to the higher TRM in studies where bone marrow was used as the source of hematopoietic stem cells. The data are further confounded by the intensity of prior therapy and patient selection. For example, in a recent Eastern Cooperative Oncology Group (ECOG) study, patients with favorable- and intermediate-risk disease received postremission therapy consisting of two courses of HiDAC followed by a course of GO and autologous HCT or autologous HCT alone. Only 49% of patients received the planned therapy, with disease progression as the most common reason for patients not proceeding to HCT. An intention-to-treat analysis demonstrated 4-year DFS and OS of approximately 35% and 41%, respectively, with no benefit from GO. Nevertheless, in favorable-risk AML, patients receiving induction that included high-dose daunorubicin who underwent autologous HCT, 4-year DFS and OS rates were 60% and 80%, respectively. For intermediate-risk patients, 4-year DFS and OS were 40% and 49%, respectively ( Fig. 3 ). These data suggest that autologous HCT may play a role in postremission therapy for a selected subgroup of patients with AML.
Maintenance Therapy
Although several trials have demonstrated an increase in DFS with maintenance therapy after intensive induction, an OS benefit has not been shown. Therefore, for younger patients, maintenance chemotherapy cannot replace more intensive postremission strategies. Immunotherapeutic approaches for maintenance therapy have also been proposed. Although several randomized studies failed to show an advantage for interleukin-2 as maintenance, a recent study showed that interleukin-2 and histamine dichloride given after the completion of consolidation therapy conferred a leukemia-free survival advantage compared with no maintenance therapy. Novel vaccination strategies are also under investigation for maintenance therapy. Wilms’ tumor antigen ( WT1 ) and proteinase 3 (PR1) peptide vaccines have elicited immune responses and produced remissions in patients with AML. Cellular therapies, such as autologous dendritic cell vaccination targeting WT1, also hold promise for postremission immunotherapy. Nevertheless, maintenance is currently not recommended outside of a clinical trial for non-APL AML.
Treatment of older patients with AML
Both patient-specific and disease-related factors contribute to the extraordinarily poor prognosis of older adults with AML. A report compiled from five SWOG trials highlighted important clinical and biologic features for younger and older patients with AML and their effect on outcome after intensive induction therapy. Although the incidence of patients with favorable cytogenetic abnormalities fell from 16% in those younger than 56 years to 4% in those older than age 75 years, the proportion of patients with unfavorable cytogenetics rose from 33% in younger patients to 50% in the older group. Similarly, the CR rate decreased from 64% in younger patients to 33% for patients older than age 75 years. The median OS durations for patients younger than age 56 and older than age 75 years were 18.8 months and 3.5 months, respectively. Although a higher rate of poor-risk cytogenetics contributed to a worse outcome for older patients, within each cytogenetic risk group, treatment outcome deteriorated with advancing age. Because of the poor prognosis of older patients with AML, many physicians are reluctant to offer intensive therapy. An analysis of Medicare recipients with AML older than age 65 years showed that because of these factors, only 30% were offered any form of intravenous chemotherapy. Nevertheless, intensive chemotherapy appears to confer a survival benefit to patients who are appropriate candidates. In a retrospective analysis of data from the Swedish Acute Leukemia Registry, outcomes were strongly dependent on age and performance status, but early death rates were lower with intensive therapy than with palliation across all age groups.
The development of prognostic models may allow clinicians to identify patients who may benefit from intensive induction chemotherapy and those who should be treated using alternative approaches. A multivariate analysis performed by investigators at the MD Anderson Cancer Center (MDACC) in patients aged 65 years and older yielded several independent poor prognostic factors, including age 75 years or older, unfavorable karyotype, poor performance status, longer duration of antecedent hematologic disorders, and abnormal organ function. Those with no adverse risk factors had expected remission rates higher than 60%, induction mortality rates of 10%, and 1-year survival rates higher than 50%. Patients with one or two risk factors had expected CR rates of 50%, induction mortality rates of 30%, and 1-year survival rates of 30%. Patients with three or more risk factors had CR rates of less than 20%, induction mortality rates of 50%, and 1-year survival rates of less than 10%. In a similar model, the MDACC group showed that most patients aged 70 years and older do not benefit from intensive induction therapy. Such predictive models, however, must be refined and validated by independent groups before integration into the design of clinical trials for new treatment regimens.
Intensive Chemotherapy
Although many strategies have been investigated to improve the outcome of induction therapy for older adults, a combination of an anthracycline and cytarabine remains the most commonly used regimen for individuals who are candidates for intensive therapy, just as in younger patients. A classic Phase III study conducted by ECOG in patients older than 55 years showed no difference in outcomes with the use of daunorubicin, idarubicin, or mitoxantrone combined with cytarabine. As with many studies in younger patients, no benefit was seen with GM-CSF priming in this trial. Although no anthracycline is clearly superior, a recent study by the HOVON, AMLSG, and SAKK groups showed that the use of higher-dose daunorubicin (90 mg/m 2 for 3 days) is feasible in patients up to age 65 years and results in improved EFS and OS compared with conventional-dose daunorubicin (45 mg/m 2 ). Despite a high incidence of multidrug resistance in older patients, the strategy of P-glycoprotein inhibition also seems of limited use in older patients with AML. SWOG investigators showed that zosuquidar, a highly selective P-glycoprotein inhibitor that does not significantly affect anthracycline clearance, did not improve OS when given with cytarabine and daunorubicin in patients older than age 60 years. The use of myeloid growth factors to reduce infectious complications and periinduction mortality during induction has only modest benefits. Both CALGB and ECOG conducted studies in which GM-CSF or placebo was given after the completion of daunorubicin and cytarabine to older patients with AML. The median duration of Grade 4 neutropenia was reduced by 2 to 4 days; however, the ECOG study showed a decreased incidence of infection-related mortality. G-CSF or placebo was studied in a similar fashion after standard induction chemotherapy. The duration of neutropenia was shortened by 6 days, but no difference in early mortality or OS was seen.
Except in patients with favorable-risk disease, postremission therapy provides only marginal benefit to older patients with AML. In addition to the good-risk cytogenetic and molecular markers discussed previously, low expression of the brain and acute leukemia, cytoplasmic ( BAALC ) gene and ets-related gene ( ERG ) may also predict older patients who benefit from intensive chemotherapy. In comparing the outcomes of older patients with AML in first remission who received two cycles of postremission therapy cytarabine (400 mg/m 2 for 5 days) with those who did not receive any postremission treatment in retrospective study, investigators from the Cleveland Clinic found no differences in DFS or OS. Most randomized studies have compared the intensity or during of postremission therapy in older individuals. The NCRI found no difference in OS after one or four courses of moderate-intensity postremission therapy. Both the AML Cooperative Group and ALFA groups found prolonged outpatient postremission therapy gave superior DFS and OS survival compared with one course of intensive consolidation. In contrast, the AMLSG found that one cycle of intensive consolidation was superior to 1 year of oral maintenance therapy. Based on these data, it is reasonable to administer one or two courses of consolidation therapy to patients without adverse prognostic markers and comorbid conditions who have a good performance status. Conversely, patients with high-risk disease derive no clear benefit from consolidation chemotherapy and should be considered for novel postremission strategies.
Hematopoietic Stem Cell Transplantation
Despite the use of nonmyeloablative or reduced-intensity conditioning regimens to decrease TRM, allogeneic HCT remains an option for only a minority of older patients. In an intention-to-treat analysis, investigators at MDACC found that only 5% of older patients with AML underwent HCT because of such issues as age, comorbid conditions, patient choice, physician attitude, and lack of a suitable donor. Most studies show HCT in older patients results in TRM in 20% to 30% with an OS rate of approximately 30%. A retrospective study from the Cooperative German Transplant Study Group suggested that match unrelated and match sibling donor HCT results in comparable survival in older patients. Current data are limited by small patient cohorts, heterogeneity of conditioning regimens, and patient selection. Ultimately, prospective randomized trials will be needed to determine if reduced-intensity conditioning HCT is superior to conventional consolidation chemotherapy in older patients.
Reduced-intensity Therapies
With the recognition that many older patients will not benefit from standard induction therapy, investigators have directed their efforts toward the development of less intensive therapeutic options ( Table 1 ). As part of the NCRI AML14 trial, 217 patients deemed unfit for standard induction chemotherapy were randomized to receive low-dose cytarabine or hydroxyurea. Low-dose cytarabine produced a higher remission rate (18% vs 1%) and OS than hydroxyurea; however, patients with adverse cytogenetics did not benefit. A variety of newer agents have also been studied in recent years with generally disappointing results. Tipifarnib, an oral farnesyl transferase inhibitor, produced remissions in 14% of elderly patients with previously untreated, poor-risk AML, and a randomized Phase III trial comparing tipifarnib with supportive care failed to show a benefit in patients aged 70 years and older. The novel alkylating agent laromustine produced responses in 32% of patients age 60 or older; however, the periinduction mortality rate was 14%, and 1-year OS was only 21%.