Autologous and allogeneic hematopoietic cell transplantation (HCT) is regularly used as a curative treatment option for patients with various disorders, including acute leukemia in adults. The past decade has witnessed dramatic improvements in the reduction of treatment-related mortality (TRM), in part attributable to improved supportive care but also due to better graft selection and donor-to-recipient matching regimens, and the emergence of reduced-intensity conditioning in place of myeloablative conditioning. Despite these advances, HCT remains plagued by the risk of relapse or failure due to graft-versus-host disease, infectious complications, and TRM. This article reviews new approaches that may improve overall patient outcome.
More than 50 years have passed from the initial report from Dr E. Donnall Thomas and colleagues describing the safety (no marrow emboli) and efficacy (some donor engraftment) of bone marrow transplanted into 6 patients with a variety of malignant and nonmalignant disorders. Worldwide, autologous and allogeneic hematopoietic cell transplantation (HCT) is regularly used as a curative treatment option for patients with various disorders, including acute leukemia. Both the chemoradiation or chemotherapy used in the preparative regimen and effects of the graft mediated via the immunologic graft-versus-leukemia (GVL) effect contribute to the success of this approach. At present, acute myeloid leukemia (AML) is the most common indication for HCT; although HCT remains the preferred therapy for a distinct subset of AML patients, acute lymphoblastic leukemia (ALL) patients are also often considered for this therapy. The past decade has witnessed dramatic improvements in the reduction of treatment-related mortality (TRM), in part attributable to improved supportive care but also due to better graft selection and donor-to-recipient matching regimens. The emergence of reduced-intensity conditioning (RIC) in place of myeloablative conditioning (MAC) broadens the applicability of this modality to patients who formerly would be excluded from HCT because of advanced age or comorbid conditions. The demonstration that the graft-versus-leukemia effect eradicates disseminated cancer has fueled efforts to develop immunotherapeutic approaches to tumors. Despite these advances, HCT remains plagued by the risk of relapse or failure due to graft-versus-host disease (GVHD), infectious complications, and TRM. New approaches are emerging that may improve overall patient outcome; many of these novel strategies are reviewed herein.
Graft sources
The earliest HCT transplants used grafts obtained from histocompatible siblings and subsequently from the patients themselves if they were in complete remission (autologous). The past decade has seen the emergence of a significant shift to the use of alternative donors, as shown in Table 1 .
| Match Probability (%) | Percentage of All Transplants | |
|---|---|---|
| HLA-compatible sibling | 30 | 54 |
| HLA-compatible unrelated donor | 35–80 | 38 |
| Umbilical cord blood | 10–90 | 5 |
| Haploidentical donor | 99 | <5 |
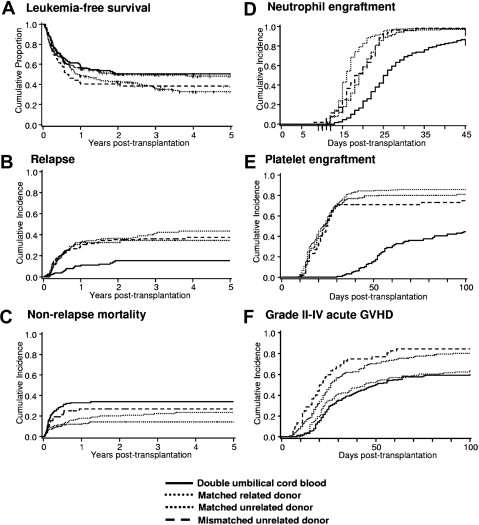
The increase in the donor pool has contributed significantly to the sustained increase in the number of AML allogeneic transplants performed in the last decade. It is hypothesized that the source of the hematopoietic progenitor cells may play a role in exerting different donor-versus-host immune effects. Fig. 1 summarizes the results of consecutive patients undergoing first allogeneic HCT from various graft sources for hematologic malignancy after a high-dose total body irradiation containing conditioning regimen at the Fred Hutchinson Cancer Research Center (FHCRC) and University of Minnesota between 2001 and 2008.
Autologous HCT
Autologous HCT is considered an alternative postremission therapy in patients with favorable-risk and intermediate-risk cytogenetics AML, whereas it cannot be recommended to patients with high-risk cytogenetics. Outcome after autologous HCT is at least as good as after the use of postremission chemotherapy, and may offer an advantage in specific subsets of AML. Fernandez and colleagues reported recently the results of a prospective, randomized phase 3 trial evaluating the use of gemtuzumab ozogamicin (GO) in an intensive consolidation approach followed by autologous HCT. A total of 657 patients with a median age of 47 years (range 17–60 years) in first complete remission (CR1) after cytarabine and standard-dose or high-dose daunorubicin induction received 2 cycles of consolidation with high-dose cytarabine followed by peripheral blood progenitor cell collection, then were randomized to receive GO (n = 132) or not (n = 138) and proceeded to autologous HCT. The subgroups of favorable-risk and intermediate-risk AML had 4-year disease-free survival rates of 60% and 40% and overall survival rates of 80% and 49.3%, respectively, while addition of a single dose of GO in this setting did not improve outcomes. For younger AML patients in CR1, autologous HCT should be considered in patients with favorable and intermediate cytogenetic risk who do not have an allogeneic donor.
This modality does not appear to have a role in ALL.
Autologous HCT
Autologous HCT is considered an alternative postremission therapy in patients with favorable-risk and intermediate-risk cytogenetics AML, whereas it cannot be recommended to patients with high-risk cytogenetics. Outcome after autologous HCT is at least as good as after the use of postremission chemotherapy, and may offer an advantage in specific subsets of AML. Fernandez and colleagues reported recently the results of a prospective, randomized phase 3 trial evaluating the use of gemtuzumab ozogamicin (GO) in an intensive consolidation approach followed by autologous HCT. A total of 657 patients with a median age of 47 years (range 17–60 years) in first complete remission (CR1) after cytarabine and standard-dose or high-dose daunorubicin induction received 2 cycles of consolidation with high-dose cytarabine followed by peripheral blood progenitor cell collection, then were randomized to receive GO (n = 132) or not (n = 138) and proceeded to autologous HCT. The subgroups of favorable-risk and intermediate-risk AML had 4-year disease-free survival rates of 60% and 40% and overall survival rates of 80% and 49.3%, respectively, while addition of a single dose of GO in this setting did not improve outcomes. For younger AML patients in CR1, autologous HCT should be considered in patients with favorable and intermediate cytogenetic risk who do not have an allogeneic donor.
This modality does not appear to have a role in ALL.
Allogeneic HCT
Bone Marrow
Bone marrow as a graft source has fallen out of favor over the past 15 years; data from the Center for International Blood and Marrow Transplant Research show that in 2008 only 12% of the related donor grafts and 14% of the unrelated donor grafts were derived from bone marrow as opposed to peripheral blood. The field anxiously awaits the results of the recently completed Blood and Marrow Transplant Clinical Trials Network (BMT CTN) prospective, randomized trial BMT CTN 0201, comparing bone marrow with mobilized peripheral blood as source of matched unrelated donor grafts.
Peripheral Blood
Dividing, nonleukemic DNA-synthesizing cells were first identified in peripheral blood in the 1950s, suggesting the existence of a small number of circulating cells of “multipotential character.” With the development of continuous-flow apheresis and the widespread implementation of hematopoietic growth factor mobilization treatment, blood rapidly replaced bone marrow as the graft source of choice. Newer strategies include the use of plerixafor, a novel small-molecule CXCR4 chemokine antagonist that enhances mobilization of hematopoietic progenitor cells from volunteer donors, with or without granulocyte colony-stimulating factor (G-CSF) (filgrastim).
Umbilical Cord Blood
This graft source first was used in 1988 in a Fanconi anemia patient who had a healthy HLA-identical sibling. The main practical advantages of using umbilical cord blood (UCB) as an alternative source of hematopoietic progenitor cells are many, including the relative ease of procurement, the absence of risks for mothers and donors, the reduced likelihood of transmitting infections, and rapid availability. Further, for a given degree of HLA match, UCB grafts are associated with a reduced degree of GVHD when compared with a matched unrelated donor graft, yet retain the GVL effect. Finally, no donor isohemagglutinins are produced in an ABO-incompatible HCT when UCB is used.
One major challenge associated with the use of UCB for transplantation is the relatively low cell dose available; this impediment contributes, at least in part, to the slower engraftment and elevated risk of engraftment failure associated with UCB HCT. Several strategies have been proposed to improve the effectiveness of UCB transplants with respect to the rates and kinetics of engraftment. These approaches include the infusion of 2 UCB units and ex vivo expansion of UCB grafts using a variety of techniques including multipotent mesenchymal stromal cells (MSCs), the copper chelator tetraethylenepentamine (TEPA), and the Notch ligand (see later discussion).
Double cord blood transplant
Whereas double UCB HCT may provide significantly more rapid neutrophil engraftment compared with use of single units, engraftment delays and failure rates remain higher when compared with bone marrow or mobilized blood sources. Furthermore, an area of continued investigation is to explain the observation of why only one unit will ultimately predominate.
In transplant recipients receiving 2 or more UCB units, one of the UCB units predominates, usually by 4 to 6 weeks after transplant. Infusion of the UCB that does not engraft may augment engraftment via immune activation, inhibition of recipient-mediated immune rejection, or the combination. On the other hand, relapse rates appear lower with 2 than with 1 unit. To date, there have been no convincing data that infusion of 2 rather than 1 UCB unit is superior; given the significantly higher cost and other issues, the standard of care remains uncertain.
Ex vivo UCB unit expansion: MSCs
De Lima and colleagues at the MD Anderson Cancer Center recently reported the early findings of a study whereby they ex vivo cocultured one of two UCB units infused in the course of a double UCB HCT. For expansion, they used either third-party haploidentical family member marrow-derived MSCs or commercially available mesenchymal progenitor cells (Angioblast Systems, Inc, New York, NY). Thirty-two patients received a MAC regimen consisting of fludarabine, melphalan, thiotepa, and antithymocyte globulin (ATG). The culture yielded a median 14-fold increase in total nucleated cells and 40-fold increase in CD34 + cells. Median time to neutrophil and platelet engraftment was 15 (range 9–42) days and 40 (range 13–62) days; there were no toxicities attributable to the expanded cells. The expanded unit contributed only to early blood cell recovery; long-term engraftment was provided by the unexpanded unit.
Ex vivo UCB unit expansion: TEPA
Peled and co-workers described a NOD/SCID mouse model in which the linear polyamine copper chelator TEPA augmented long-term ex vivo expansion of UCB-derived CD34 + cells and increased their engraftment potential. Subsequently, the MD Anderson Cancer Center group conducted a clinical trial in which 10 high-risk hematologic malignancy patients (n = 5 ALL; n = 2 AML) received high-dose cytotoxic therapy followed by infusion of a UCB unit that had been cryopreserved in 2 fractions. The GVHD prophylaxis regimen was combination tacrolimus with “mini-methotrexate” (3 days). One of the fractions was administered unexpanded, whereas the other component was CD133-selected and expanded in vitro in media containing stem cell factor (SCF), FLT-3 ligand, interleukin (IL)-6, thrombopoietin, and TEPA. The total nucleated cell dose in the manipulated component was expanded 219-fold and the CD34 + cell content increased sixfold. Despite the fact that the mean total nucleated cell content infused was low (1.8 × 10 7 /kg), 9 of 10 patients engrafted at times comparable with those associated with infusion of unmanipulated single UCB units, for example, neutrophils at 30 days and platelets at 48 days. These data demonstrate that a graft with low cell numbers can be used successfully and justify exploring this approach in a larger patient series, in which methotrexate should be omitted from the GVHD prophylaxis regimen.
Ex vivo UCB unit expansion: Notch ligand
The Notch signaling pathway has been known to play an important role in hematopoiesis. Hence, the investigators at the FHCRC initiated a phase 1 MAC UCB HCT trial in 10 high-risk acute leukemia patients who were given one nonmanipulated and one ex vivo manipulated UCB graft. One UCB unit was thawed and the CD34 +– selected cells were expanded in serum-free media containing the Notch ligand Delta1 supplemented with SCF, thrombopoietin, FLT-3, IL-3, and IL-6. CD34 + cells were expanded (mean) 164-fold, and median (range) time to attain neutrophil recovery greater than 500/μL was 16 (range 7–34) days; these numbers compare favorably with values of 26 (range 16–48) days ( P = .002) from a concurrently treated cohort of 20 patients undergoing double UCB transplantation.
These novel UCB expansion projects are the subject of several investigations in various stages, undertaken by academic centers as well as corporate partners.
Haploidentical Bone Marrow
Given the limited numbers of matched related donors and difficulties in identifying and obtaining matched unrelated donor grafts, several investigators have pursued HCT using haploidentical relatives as donors. In this setting the donor could be a sibling, parent, or child. The initial trials were reported from the center in Perugia, Italy ; this group used T-cell–depleted peripheral blood progenitor cell grafts that were associated with a low rate of GVHD. This approach, however, was hampered by a high rate of graft rejection, slow immune recovery, and substantial TRM, often due to opportunistic infection. The group at Johns Hopkins uses unmodified haploidentical marrow followed by posttransplantation treatment with cyclophosphamide. The thought is that donor alloreactive T cells will be eliminated during their proliferative phase early after infusion into the recipient. Their data show a lower rate of severe acute and chronic GVHD and TRM, but engraftment failure rates are higher.
More recently, The BMT CTN conducted two parallel, multicenter, phase 2 RIC trials for leukemia and lymphoma patients who lacked suitable related donors. The graft source was either unrelated double UCB or HLA-haploidentical related donor bone marrow transplantation. For both trials, the conditioning regimen incorporated cyclophosphamide, fludarabine, and single-fraction 200 cGy total body irradiation (TBI). The 1-year probabilities of overall and progression-free survival were 54% and 46%, respectively, after double UCB transplantation (n = 50) and 62% and 48%, respectively, after haploidentical marrow transplantation (n = 50). Neutrophil recoveries were similar, and 100-day cumulative incidence of grade II to IV acute GVHD was 40% after double UCB and 32% after haploidentical marrow transplantation. Nonrelapse mortality and relapse incidence at 1 year after double UCB transplantation were 24% and 31%, respectively, with corresponding results of 7% and 45%, respectively, after haploidentical marrow transplantation.
These studies broaden the range of HCT, as almost every patient can count on an adequately matched UCB or haploidentical donor. Further work is needed to directly compare these approaches and optimize their outcome.
Paradigms in conditioning
The intensity of the chemoradiation or chemotherapy used for the conditioning (or preparative) regimen has been a source of continuous debate in the area of transplant efficacy. With greater understanding of patient tolerance to therapy, the approach to the patient has resulted in the recognition of 3 large categories of preparative regimens: MAC, RIC, and nonmyeloablative conditioning (NMA) regimens. In fact, for the latter (NMA) approach, prompt hematologic recovery could be expected within 28 days without hematopoietic progenitor cell rescue, often with resulting mixed chimerism. Various NMA or RIC regimens have been used as an alternative to MAC to reduce toxicity in patients with an impaired performance status or in those subjects with visceral organ compromise. RIC and NMA HCT rely more on the allogeneic effect of the infused graft mediating a GVL effect rather than the antitumor effect of high-dose chemotherapy. RIC regimens continue to be hindered by the morbidity and mortality of GVHD. The details of many of these approaches have been published elsewhere.
No one regimen has been demonstrated to be superior, and most centers rely on the conditioning with which they have generated the most experiences. So far, it has been established that survival in older patients after RIC HCT is comparable with that in younger patients, with no significant differences in nonrelapse mortality and overall survival between patients older than 55 and those younger than 55 years; as expected, the data show significantly lower 1-year infection-related mortality after RIC than after MAC.
Novel Preparative Regimens: Chemotherapeutic Agents
Taking advantage of the new developments in pharmacotherapy, several investigators have initiated newer approaches.
Clofarabine-based
Clofarabine, a new potent purine nucleoside antimetabolite that inhibits DNA polymerase and ribonucleotide reductase, is approved by the Food and Drug Administration for the treatment of relapsed or refractory childhood ALL. Biochemical and clinical studies suggest synergy with cytarabine and significant activity in acute leukemia; hence, this agent has been combined in preparative regimens. Ricci and colleagues reported a combination of clofarabine, cytarabine, and TBI followed by allogeneic HCT in 12 poor-risk children, adolescents, and young adults with acute leukemia. Clofarabine was dose-escalated to the maximum tolerated dose of 52 mg/m 2 /d for 5 days. TRM at 100 days was 0%; 2 patients had progressive disease while 8 patients remain alive in continuous complete remission at a median (range) of 209 (40–770) days. At the 2011 BMT tandem meeting, Agura and colleagues described preliminary results of a single-institution phase 2 clofarabine and busulfan RIC regimen in 20 hematologic malignancy (n = 16 AML) patients. Eleven subjects with active malignancy attained complete remission by day 30; 7 experienced relapse and overall, 12 died but 6 remain in remission at a median (range) follow-up of 31 (13–41) months. Kirschbaum and colleagues performed a phase 1 dose-escalation study of clofarabine and melphalan in 16 AML patients, median age 63 (range 30–66) years. The investigators did not identify dose-limiting toxicities; 2 patients expired early from multiorgan toxicities. Only 2 subjects relapsed and 11 remain in remission at a median (range) of 23 (4–35) months after HCT. The regimen of 5 days of clofarabine 40 mg/m 2 /d plus 1 day of melphalan 100 mg/m 2 appears to be an effective combination worthy of additional study. These clofarabine-containing regimens appear to be relatively well tolerated and show promising antileukemic activity.
Treosulfan-based
Treosulfan, pharmacologically similar to busulfan but more potent in vitro, is a bifunctional, alkylating agent with myeloablative as well as immunosuppressive properties. Unlike busulfan, treosulfan does not appear to require dose adjustment and has an excellent toxicity profile. Furthermore, in contrast to busulfan, treosulfan is active against both primitive and committed hematopoietic progenitor cells. Danylesko and colleagues recently reviewed this agent in HCT. Several interesting studies reported included a prospective, multicenter RIC trial from France that combined treosulfan (12 mg/m 2 /d for 3 days) with fludarabine (30 mg/m 2 /d for 5 days) and ATG (2.5 mg/kg/d for 2 days) in 56 hematologic malignancy patients. Therapy was well tolerated and median overall survival was not reached at a median follow-up of 13 months, with a 52% 3-year probability of survival.
Nemecek and colleagues undertook a prospective, multicenter trial in 60 patients (n = 44 AML) at high risk for relapse or TRM. Median (range) age was 46 (5–60) years. Therapy consisted of treosulfan 12 to 14 mg/m 2 /d for 3 days and fludarabine 30 mg/m 2 /d for 5 days, followed by HCT from HLA-identical siblings (n = 30) or unrelated donors (n = 30). All patients engrafted, and the 2-year nonrelapse mortality was 8%. With a median follow-up of 22 months, 2-year relapse-free survival for all patients was 58% and 88% for patients without high-risk cytogenetics. The cumulative incidence of relapse at 2 years was 33%. Newell and colleagues reported the results of a single-institution double UCB HCT protocol in 15 hematologic malignancy patients aged 4 to 63 (median 48) years including 7 AML subjects. Conditioning consisted of a regimen of treosulfan 14 mg/m 2 /d for 3 days, fludarabine 30 mg/m 2 /d for 5 days, and low-dose TBI 200 cGy. Median time to neutrophil recovery was 22.5 days and only one patient failed to engraft. Treosulfan appears to be an effective and well tolerated, interesting new addition to the HCT armamentarium, although there are no clinical HCT studies directly comparing this agent with busulfan alone or in combination.
Plerixafor and G-CSF
Plerixafor recently has been incorporated into conditioning regimens. The marrow microenvironment contributes to leukemia cell chemoresistance for those cells residing in niches. Preclinical models indicate that inhibition of the chemokine receptor CXCR4 will mobilize leukemia cells into the circulation and facilitate sensitization to cytotoxic therapy. Konopleva and colleagues at the MD Anderson Cancer Center reported in preliminary fashion a phase 1/2 study in which G-CSF and plerixafor therapy were initiated in escalating dose before the start of a standard fludarabine and busulfan regimen. The investigators showed preferential mobilization of clonal leukemia cells over normal cells in this 27-patient study, without toxicities ascribed to the G-CSF/plerixafor treatment. This approach will require validation in a larger series and longer follow-up to determine efficacy.
Novel Preparative Regimens: Radiation
Selective radiation approaches
While TBI is used to prevent immunologic graft rejection and can eradicate residual malignant cells, toxic effects may be significant, and include cardiac toxicity, pulmonary fibrosis, renal failure and, especially in the pediatric population, an excess number of secondary malignancies. Some investigators have attempted to improve on TBI using helical tomotherapy, a modality that integrates computed tomography image-guided radiotherapy and intensity-modulated radiation therapy into a single device. This technique can deliver conformal targeted radiation to the marrow and lymphatic systems. Rosenthal and colleagues recently described the results of a phase 1/2 investigation using total marrow and lymph node irradiation (TMLI) to augment RIC transplantation for 33 advanced, poor-risk hematologic malignancy patients with a median age of 55 years. The addition of TMLI 1200 cGy (administered as 150 cGy/fraction in 8 fractions over 4 days) to fludarabine 25 mg/m 2 /d for 5 days and melphalan 140 mg/m 2 /d for 1 day, did not appear to increase toxicity of this chemotherapy preparative regimen. At 1 year after HCT, overall survival, event-free survival, and nonrelapse-related mortality rates were 75%, 65%, and 19%, respectively. The addition of TMLI to RIC is feasible and safe, and the centers equipped with this device can offer it to patients with advanced hematologic malignancies who might not otherwise be candidates for RIC.
Targeted myeloablative radioimmunotherapy
An alternative strategy for acute leukemia patients undergoing HCT is myeloablative radioimmunotherapy (RIT), a technique that targets radiotherapy to the bone marrow using radiolabeled monoclonal antibodies. Candidate isotopes usually are the β-emitter radionuclides ( 131 I, 90 Y, 188 Re), which are conjugated to monoclonal antibodies including anti-CD33, anti-CD45, and anti-CD66. The cross-firing β particles create a field radiation effect, potentially killing antigen-negative resident marrow tumor cells. Most clinical studies have used 131 I, a long-lived β-particle emitter, whose γ emissions allow dosimetry studies to be performed easily, but require patient isolation. 90 Y is a pure β emitter and the absence of γ emission allows outpatient administration of high doses, but dosimetry calculations are extremely complex. 188 Re is another radioisotope suitable for biodistribution and dosimetry as it also has γ emission. On the other hand, α particles are helium nuclei emitted from the decay of radioisotopes. RIT using α emitters, such as 213 Bi, 225 Ac, and 211 At, should result in less nonspecific toxicity to normal bystander cells and provide a more efficient single-cell killing than β-emitting constructs. Clinical experiences have been very limited.
Although the efficacy of RIT depends on a variety of factors, the most important appears to be biodistribution. Kletting and colleagues described physiologically based pharmacokinetic models to estimate biodistribution and maximize anti-CD45 antibody and anti-CD66 RIT efficacy. These approaches have not yet penetrated clinical practice. On the other hand, Schulz and colleagues recently reported an open-label, single-center pilot study of 30 pediatric and adolescent patients undergoing HCT for malignant (n = 16) and nonmalignant (n = 14) disorders using treatment with a 90 Y-labeled anti-CD66 monoclonal antibody. This patient population was considered at high risk for nonrelapse mortality due to advanced disease, ongoing severe infections, second HCT, and significant visceral organ damage. The therapeutic 90 Y activity was calculated to deliver a marrow dose of 3000 to 4000 cGy to patients in the malignant group and 1600 to 2000 cGy in the nonmalignant group. Patients also received additional conditioning with either TBI-based or intravenous busulfan, melphalan, and fludarabine-containing therapy. No excess acute organ toxicity was observed in any of the patient groups. Twenty-three patients are alive at a median (range) of 35 (19–71) months after HCT. The overall nonrelapse mortality in this extremely poor-risk group was only 13%, and supports continued exploration of this approach.
Pretargeted myeloablative radioimmunotherapy
While the use of radiolabeled antibodies has shown promise in both imaging and therapeutic applications, the clinical adoption has not fulfilled the original expectations, due either to poor image resolution and contrast in scanning, or to insufficient radiation doses delivered selectively to tumors for therapy. Pretargeting involves the separation of the localization of tumor with an anticancer antibody from the subsequent delivery of the imaging or therapeutic radionuclide.
Pretargeted RIT (PRIT) circumvents the issue of suboptimal therapeutic index (target-to-nontarget ratio) by separating the prolonged-circulating antibody construct (“targeting vehicle”) from the therapeutic radioisotope (“effector”). Conceptually, this sequential administration allows for maximal antibody targeting to take place prior to delivery of the therapeutic radionuclide while maintaining targeting specificity. PRIT substantially reduces whole-body radiation because of radionuclide delivery via small molecules that yield rapid tumor uptake and fast renal excretion of nontumor-bound radioactivity. Synthetic chasers (“clearing agents”) have been introduced as an additional refinement to PRIT. At present, this technique has not yet been implemented in clinical practice but holds considerable promise as a means of enhancing the therapeutic index of RIT.
GVHD prevention strategies
The prevention of GVHD remains an elusive goal in the quest to improve overall patient outcome after HCT. For many decades, numerous investigators have used either administration of pharmacologic agents to the patient or manipulation of the graft. A full discussion of GVHD prophylaxis is beyond the scope of this review, and the authors focus here on graft manipulation.
T-Cell Depletion of the Graft
Although T-cell depletion strategies have resulted in lower rates of acute GVHD, these techniques have unfortunately met high engraftment failure, increased post-HCT relapse rates, inability to reduce chronic GVHD rates, high cost, and the need for special expertise.
Soiffer and colleagues reported on whether immune modulation with anti–T-cell antibody infusion abrogates the therapeutic benefits of transplantation in a RIC setting. The study included 1676 patients aged 21 to 69 years with ALL, AML, chronic myeloid leukemia (CML), myelodysplastic syndrome, chronic lymphocytic leukemia (CLL), non-Hodgkin lymphoma, and Hodgkin lymphoma. All patients received alkylating agent plus fludarabine; 792 received allografts from an HLA-matched sibling donor, 884 from an HLA-matched unrelated donor. Outcomes after in vivo T-cell depletion (n = 584 ATG; n = 213 alemtuzumab) were compared with T-cell–replete (n = 879) transplantation. Grade 2 to 4 acute GVHD was lower with alemtuzumab than with ATG or T-cell–replete regimens (19% vs 38% vs 40%, P <.0001), and chronic GVHD was lower with alemtuzumab and ATG regimens in comparison with T-cell–replete approaches (24% vs 40% vs 52%, P <.0001). However, relapse was more frequent with alemtuzumab and ATG than with T-cell–replete regimens (49%, 51%, and 38%, respectively, P <.001). Disease-free survival was lower with alemtuzumab and ATG than with T-cell–replete regimens (30%, 25%, and 39%, respectively, P <.001). Corresponding probabilities of overall survival were 50%, 38%, and 46% ( P = .008). These data suggest adopting a cautious approach to routine use of in vivo T-cell depletion with RIC regimens.
Recently, Devine and colleagues within BMT CTN performed a phase 2 single-arm multicenter study in 44 AML CR1 (n = 37) and CR2 (n = 7) patients with a median (range) age of 48.5 (21–59) years. These investigators administered MAC chemotherapy, fractionated TBI (1375 cGy), and immune-magnetically selected CD34-enriched, T-cell‒depleted allografts from HLA-identical siblings. No pharmacologic GVHD prophylaxis was given. All patients engrafted; the incidence of grade II to IV acute GVHD was 22.7%, and the incidence of extensive chronic GVHD at 24 months was 6.8% with a 17% relapse rate at 36 months for CR1 patients. Disease-free survival for CR1 patients was 72.8% at 12 months and 58% at 36 months. The BMT CTN investigators demonstrated that a MAC HCT can be performed in a multicenter setting using a uniform method of T-cell depletion that results in a low risk of extensive chronic GVHD and relapse for AML patients in CR1. Such a strategy should be studied in a comparative fashion.
Treg Therapy for GVHD Prevention
Forty years ago Gershon and Kondo discovered the so-called suppressor T cells, a subpopulation of T cells that dampen the immune response. The field was reignited in 1995 when Sakaguchi and colleagues, using a murine model, identified CD4 + T cells coexpressing CD25 (the IL-2 receptor α chain) as a thymus-derived population of peripheral T cells capable of inhibiting autoimmunity otherwise resulting from neonatal thymectomy. Secondary transfer of murine T cells depleted of the CD4 + CD25 + subpopulation resulted in systemic autoimmunity, including a “graft-versus-host like wasting disease.” Further models suggested that donor regulatory T cells (Tregs) can reliably suppress GVHD, possibly sparing leukemia-specific immune responses. In the milieu of preclinical promise, Brunstein and colleagues reported the “first-in-human” clinical trial of ex vivo expansion of CD4 + CD25 + FoxP3 + T regulatory cell enrichment from cryopreserved UCB. Twenty-three patients with a median (range) age of 52 (24–68) years were given intravenous cyclophosphamide 50 mg/kg, intravenous fludarabine 40 mg/m 2 /d for 5 days, single-fraction TBI 200 cGy, and double UCB HCT followed by the expanded cell population. No infusional toxicities were observed and UCB Tregs were detected for up to 14 days. When compared with 108 identically treated historical controls who did not receive Treg infusions, the incidence of grade II to IV acute GVHD was reduced from 61% to 43% ( P = .05). This maneuver was not associated with deleterious effects on risks of infection, relapse, or early mortality, and merits further consideration.
Di Ianni and the Perugia group evaluated the impact of early infusion of Tregs, followed by conventional T cells (Tcons), on GVHD prevention and immunologic reconstitution in 28 high-risk hematologic malignancy patients (n = 22 AML) who underwent HLA-haploidentical HCT after single-fraction TBI 800 cGy, thiotepa 4 mg/kg/d for 2 days, cyclophosphamide 35 mg/kg/d for 2 days, and fludarabine 40 mg/m 2 /d for 5 days. These investigators showed for the first time in humans that adoptive transfer of Tregs prevented GVHD in the absence of posttransplantation immunosuppression, as only 2 patients developed grade II or greater acute GVHD and no patient developed chronic GVHD. Although 13 subjects died of infection or visceral organ injury, at a median follow-up of 12 months, 12 patients remain alive at a median (range) of 12 (9–21) months after haploidentical HCT. This approach clearly is associated with the need for considerable expertise and is hampered by high rates of opportunistic infection.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree