Summary of Key Points
- •
Low-dose multidetector computed tomography (CT) screening of active and former heavy smokers has been shown to decrease lung cancer mortality.
- •
The malignant potential of solid pulmonary nodules depends on their size, with larger nodules more likely to represent tumor. Nevertheless, solid nodules have a low overall malignancy rate (7%).
- •
Ground-glass nodules and part-solid nodules have a higher chance of malignancy than completely solid nodules, up to 63% in the case of mixed density solid and ground-glass lesions. The differential diagnosis for ground-glass lesions that persist after 3 months includes focal fibrosis, atypical adenomatous hyperplasia, and indolent adenocarcinoma.
- •
Chest CT with intravenous contrast is recommended for all patients with suspected or known lung cancer to assess primary tumor characteristics, nodal disease, and intrathoracic or extrathoracic metastases.
- •
Accurate staging of lung cancer is critical to inform management decisions and therapy. The seventh edition of tumor, node, and metastasis (TNM) staging from the International Association for the Study of Lung Cancer/American Thoracic Society is currently in use; proposed revisions for the eighth edition have been published.
- •
Magnetic resonance imaging (MRI) is useful in evaluating several factors that determine potential resectability of lung tumors including pericardial or myocardial invasion, brachial plexus involvement in the setting of superior sulcus tumors, and invasion of regional vasculature or the spinal cord in central tumors.
- •
MRI is also showing promise in detection of metastatic lymph nodes, displaying better sensitivity and accuracy compared with 18 F-2-deoxy- d -glucose-positron emission tomography–CT in some studies.
- •
Chest CT with contrast is useful in assessing lung cancer response to radiation or chemotherapy, surgical resection, and emergent conditions that may be associated with malignancy.
Primary lung cancer is the leading cause of cancer mortality worldwide and constitutes a major public health challenge. In the United States, lung cancer is as deadly as the next three causes of cancer deaths combined (prostate, colorectal, and pancreatic cancer in men; breast, colorectal, and pancreatic cancer in women). In 2014, the incidence of lung cancer is estimated to be 224,210 cases, with lung cancer responsible for 86,930 deaths in men and 72,330 deaths in women in the United States. In 2013 in the European Union, primary lung cancer caused approximately 187,000 deaths in men and 82,640 deaths in women, the latter constituting a 7% increase from 2009. Nevertheless, the lung cancer epidemic is considered to have peaked in the developed world, and more than half of new lung cancer cases now occur in developing countries. In countries such as China, where widespread tobacco smoking has become a more recent event, lung cancer incidence and mortality rates are increasing.
Primary carcinoma of pulmonary origin includes several distinct histologic types and may be divided into small cell lung cancer (SCLC) and nonsmall cell lung cancer (NSCLC). SCLC is a pulmonary neuroendocrine tumor with aggressive features. Other neuroendocrine malignancies of the lung are carcinoid and large cell neuroendocrine tumors. NSCLC is more common overall than SCLC and encompasses adenocarcinoma and squamous cell carcinoma, as well as less frequently seen tumors of the lung such as large cell carcinoma, sarcomatoid carcinoma, and spindle cell sarcoma.
A shift in the prevailing lung cancer cell type occurred in the latter decades of the 20th century. In the 1950s, cases of squamous cell carcinoma outnumbered cases of adenocarcinoma, the second most common type of lung cancer, by a ratio of 17:1. Since that time, polycyclic aromatic hydrocarbons, a known carcinogen specifically associated with squamous cell carcinoma of the lung, have been reduced in manufactured cigarettes, with a relative decline in the incidence of squamous cell carcinoma. However, the incidence of primary lung adenocarcinoma has increased during the same period. These cancers have been linked to tobacco-specific nitrosamines, which are still present in cigarettes in substantial quantities. The use of cigarette filters, although reducing the risk of squamous cell carcinoma, has had no effect on the risk of adenocarcinoma.
Radiologic Presentation of Lung Cancer
Diagnostic imaging plays an important role in the diagnosis, workup, and staging of primary lung cancer. Conventional chest radiographs and computed tomography (CT) of the chest both have a role in identifying and evaluating abnormalities in the thorax. Chest CT is an essential tool in tumor staging and in determining clinical and surgical management.
On imaging, primary lung cancers vary in their appearance from a solitary pulmonary nodule to amorphous consolidation. They may also have varying densities, ranging from ground-glass attenuation (defined as slightly increased lung density through which vessels can be seen), to mixed ground-glass and solid density lesions, to solid tumors. A lung cancer may be cavitary at presentation or may cavitate during the course of treatment. The early, common, and uncommon imaging features of lung cancer are discussed in this chapter.
Solitary Pulmonary Nodule Characterization
Many lung cancers, particularly at the early stages, present as a solitary pulmonary nodule (up to 3 cm) or mass (greater than 3 cm), but only a small fraction of pulmonary nodules are actually cancerous. Several features seen on imaging may help to differentiate malignant from benign nodules, the most important of which are size, density, and enhancement. Other important characteristics include border contour, shape, patterns of calcification, presence of macroscopic fat, and cavitation ( Fig. 21.1 ). Location and multiplicity of nodules should also be noted.
Size
The larger a nodule is at presentation, the higher the likelihood that it is malignant. For a solid, discrete pulmonary nodule, the risk of cancer is categorized according to size. A solid nodule up to 5 mm has a 1% risk of being cancer. Nodules between 6 mm and 10 mm have a 24% chance of malignancy, which increases to 33% for nodules 11 mm to 20 mm. Solid lesions greater than 20 mm in diameter have an 80% chance of malignant histology. A lesion greater than 30 mm has a 93% to 99% rate of malignancy.
Changes in nodule size are also an important prognostic consideration. Nodules that decrease in size over time are most likely infectious or inflammatory in etiology. Conversely, nodules that enlarge while under observation are considered malignant until proven otherwise. Consequently, established algorithms for managing pulmonary nodules are promulgated by the Fleischner Society. For follow-up of solid nodules, slight variations may exist, depending on the risk stratification of the individual. In a high-risk person such as a smoker, nodules up to 4 mm should have a single follow-up 12 months after presentation. Solid nodules greater than 4 mm and up to 6 mm should be reassessed by CT at 6 months to 12 months after presentation and then at 18 months to 24 months if unchanged. For nodules greater than 6 mm to 8 mm, the initial follow-up is at 3 months to 6 months, followed by reassessment at 9 months to 12 months and again at 24 months, if unchanged. Nodules greater than 8 mm may be followed-up with CT at 3 months, 9 months, and 24 months; alternatively, dynamic contrast-enhanced CT, positron emission tomography (PET), and/or biopsy could be performed. Solid nodules identified on presentation should be followed up for a minimum of 2 years to establish stability and benignity. Interval nodule growth should prompt further workup, including biopsy.
Density
Solid nodules have a low overall malignancy rate of 7%. Subsolid nodules have a greater chance of being malignant than solid nodules and may represent primary adenocarcinomas of the lung.
Ground-Glass Opacity
Pure ground-glass lesions, those lesions with homogeneous transparent density through which underlying architectural features are seen, have an 18% chance of being malignant. Very small ground-glass nodules, up to 5 mm, may reflect atypical adenomatous hyperplasia, which is premalignant and does not require CT follow-up, according to the Fleischner Society recommendations for subsolid nodules. For lesions greater than 5 mm, the differential diagnosis includes focal fibrosis, atypical adenomatous hyperplasia, and indolent primary adenocarcinoma. Neoplasms with pure ground-glass attenuation usually correspond with adenocarcinoma in situ, the type A lesion in the Noguchi classification scheme of pulmonary adenocarcinomas, although rarely, they may be of mixed subtype. Heterogeneous ground-glass lesions or lesions with internal alveolar collapse are compatible with Noguchi type B lesions. Ground-glass adenocarcinomas have doubling times on the order of 384 days to 567 days.
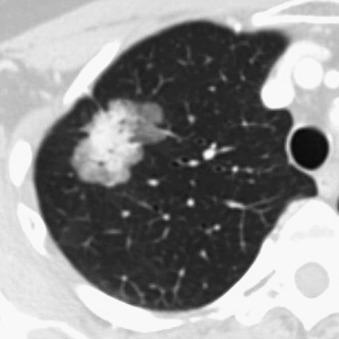
The presence of solid components mixed with ground-glass attenuation denotes a mixed-density, part solid nodule (PSN). These part solid and part ground-glass nodules have the highest malignancy rate (63%). The larger size of the solid features is associated with worse prognosis; these lesions correspond to Noguchi types B and C ( Fig. 21.2 ). The development of solid density within a pure ground-glass opacity (GGO) under surveillance is an indication of transformation to a more aggressive lesion. An increase in diameter in an observed GGO is also a sign of progression ( Fig. 21.3 ). Papillary, tubular, acinar, and other subtypes of lung adenocarcinoma on imaging cannot be reliably distinguished; rather, the diagnosis is histologic.
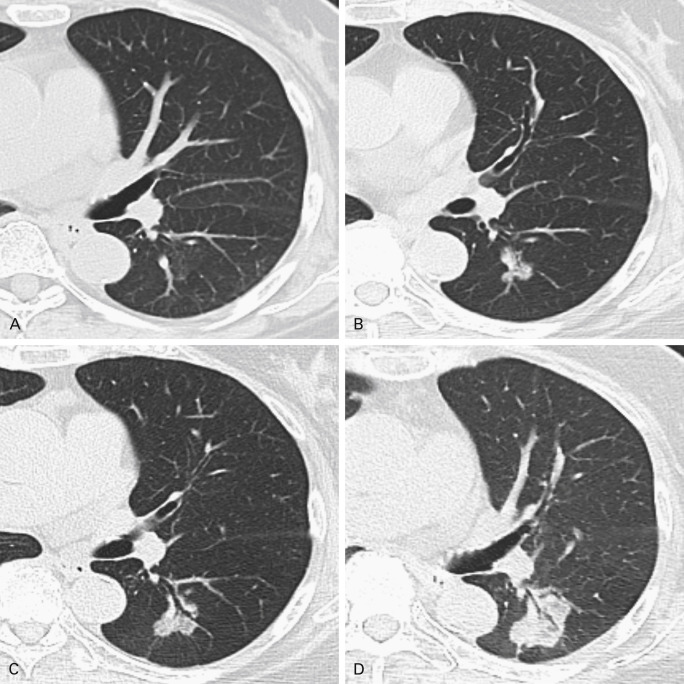
Initial follow-up for both pure GGOs and PSNs is performed 3 months after presentation to distinguish a potentially infectious or inflammatory lesion, which would be expected to decrease or resolve in this period. Single GGOs greater than 5 mm in diameter that persist after 3 months without change should be reassessed annually for a minimum of 3 years, unless or until they show progression. Solitary PSNs are managed according to the size of the solid component. If the solid portion is less than 5 mm, follow-up for the nodule may be similar to that for a GGO, for a minimum of 3 years. Lesions with larger solid components persistent after 3 months should be reassessed by biopsy and/or resected.
The presence of several GGOs and/or mixed-density lesions makes resection of all lesions impossible for some individuals. Multiple GGOs 5 mm or less should have a follow-up CT at 2 years and again at 4 years after presentation, in the absence of interval changes. Pure GGOs greater than 5 mm without a dominant lesion should undergo annual surveillance similar to that for a single GGO. Careful follow-up of these GGOs should be done in order to identify candidate tumors for limited resection based on progression or aggressive transformation. When a dominant lesion or lesions with part solid components are present in the setting of other nodules, biopsy and/or lung-sparing surgical resection is advised.
Enhancement
Neoplastic pulmonary nodules have been shown to have increased vascularity compared with benign nodules, suggesting that nodule enhancement can be used as a distinguishing characteristic in evaluating indeterminate solid lesions. The assessment process requires noncontrast CT images through the pulmonary nodule, which is then imaged at specific time points up to 4 minutes after administering intravenous contrast medium. Hounsfield unit (HU) attenuation measurements are taken with a region of interest for every time point and the differential enhancement is calculated. A difference of 15 HU or less is strongly suggestive of benignity. With a 15-HU threshold, investigators have found 98% sensitivity but 58% specificity for malignancy. Another study using a threshold of 30 HU with multidetector CT demonstrated 99% sensitivity for malignancy, with 54% specificity, a positive predictive value of 71%, and a negative predictive value of 87%. Nevertheless, because of time constraints, need for technical expertise, availability of other noninvasive assessment methods such as PET–CT, and radiation dose to the patient, nodule enhancement studies are not frequently performed in the nonacademic clinical setting. More recently, dual-energy CT, which has the capability of creating a virtual noncontrast image, has been investigated as a potential method of measuring nodule enhancement without multiple acquisitions, thus minimizing the radiation dose.
Borders
A nodule with an irregular or shaggy border can raise suspicion for malignancy; however, infectious and inflammatory nodules may have a similar appearance. The degree of suspicion may rest on the overall constellation of radiographic findings, the individual’s symptoms and demographic characteristics, and persistence over time. Cancers may be multilobulated, although smooth and well-circumscribed nodules may be malignant 21% of the time. Spiculation in the periphery of a nodule correlates pathologically with a desmoplastic reaction of the nodule or tumor infiltration into the surrounding lung parenchyma and is present much more frequently in malignant nodules. Many lung cancers are ill defined, hindering detection on radiographs.
Shape
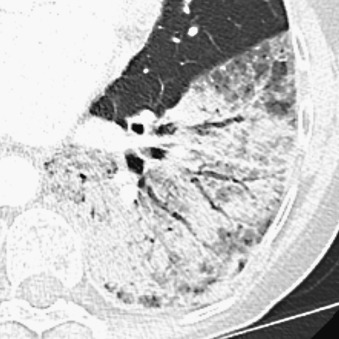
Although most lung cancers manifest as a nodule or mass, the overall shape of carcinomas varies. Some adenocarcinomas, particularly the mucin-producing subtype, can present as ill-defined parenchymal consolidation caused by mucin filling the alveoli; this is radiographically indistinguishable from pneumonia but will persist despite antibiotic treatment ( Fig. 21.4 ). An uncommon manifestation of early lung cancer is a focally thickened or impacted bronchus, sometimes with peripheral inflammatory changes.
Calcification
Calcifications within a nodule can be evaluated according to their pattern. Benign calcifications are central, diffuse, laminar, or so-called popcorn in shape. Eccentric calcification is associated with malignancy. Nonetheless, preexisting calcifications in the lungs such as granulomas may become engulfed by a tumor, and carcinoid tumors can have punctate calcifications, making the presence of calcification less reliable in determination of benign disease.
Adipose Content
Macroscopic fat within a pulmonary nodule can be identified visually and is measurable with a region of interest; HU measurement less than 1 is compatible with adipose tissue. The presence of macroscopic fat is an indication of benignity and favors the diagnosis of hamartoma, a smooth muscle neoplasm having no malignant potential. However, low attenuation in a nodule without the presence of actual fat can suggest necrosis or the presence of mucin.
Cavitation
Up to 22% of primary lung cancers demonstrate cavitation on CT imaging. Squamous cell carcinomas of the lung are the most likely to exhibit this feature; however, primary lung adenocarcinomas will also cavitate. One type of cavitation occurs when tumors outgrow their blood supply and undergo central necrosis. Some adenocarcinomas exhibit a phenomenon of pseudocavitation caused by bronchial or alveolar expansion within the tumor ( Fig. 21.5 ). It is also possible for a lung cancer to arise in the wall of a preexisting cystic space.
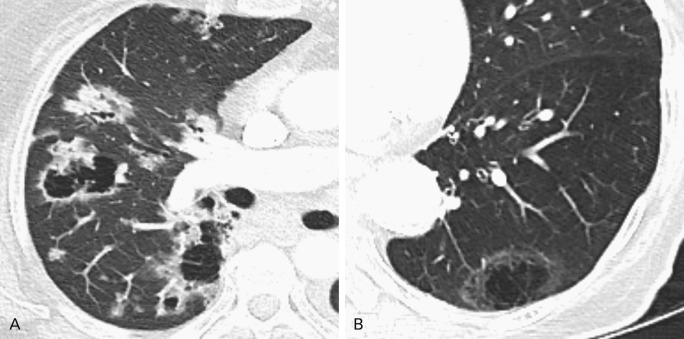
Nevertheless, infectious processes of the lung, including mycobacterial, fungal, and bacterial pneumonias, can also cavitate, as can nodules related to vasculitis. Some early studies demonstrated that cavity wall thickness measured on radiographs could be a discriminating feature in distinguishing nonmalignant from malignant disease; however, results of other studies using CT have suggested that measuring wall thickness is not as useful. Both inner cavity wall irregularity and notching, or focal lobulation of the outer cavity wall, have both been shown to occur more frequently in malignant than in benign cavitary lesions.
Multiplicity
The implication of more than one lesion also depends on size and density. Multiple small, solid nodules that are smaller than 6 mm are most likely to be postinfectious/postinflammatory and are considered low risk for malignancy. Conversely, numerous ground-glass nodules and/or PSNs are highly suggestive of multifocal synchronous primary adenocarcinomas. The presence of two synchronous primary lung carcinomas of different histologic types is also possible but uncommon.
Location
Lung cancers may be located in the peripheral or central aspect of the lungs. Statistically, more malignancies are found in the upper lobes, especially the right upper lobe.
Imaging with Chest Radiographs
The chest radiograph is by far the most commonly performed radiographic study, with almost 80.5 million chest radiographs performed in 2010 in short-term-stay US hospitals alone, not including associated facilities, specialty hospitals, and independent doctors’ offices. Radiographs are inexpensive and widely available, and the radiation dose is negligible (0.1 rem). Chest radiography is the first-line imaging modality for individuals with symptoms referable to the chest, such as cough, shortness of breath, hemoptysis, and chest pain. Chest radiographs are also obtained as baseline examinations before procedures, including surgery. The chest radiograph, therefore, provides an early opportunity to detect both symptomatic and asymptomatic lung cancers.
The error rate for the detection of lung cancer by chest radiography is generally accepted to be 20% to 50%, which is likely the result of a combination of factors. Even with an adequate search pattern and search duration, limitations in human sight, technical aspects of the radiograph, and tumor characteristics can make identification of an early lung cancer challenging.
Technical Factors
The radiograph itself may be of limited quality for a variety of technical reasons, including overpenetration or underpenetration of the chest by the photon beam or positioning variances such as rotation or lordosis. Objects external to the patient may cause artifacts. Although metal objects such as jewelry and clothing fasteners are easy to identify, nonmetal items such as buttons, hair, or clothing decorations may be confounding. Parts of the lung may be excluded from the field of view by faulty technique. Lastly, the ability of the patient to achieve full lung inspiration on the radiograph affects the conspicuity of lesions. Visualization of nodules is enhanced by using higher peak kilovoltage (140 kVp), which accentuates contrast.
Blind Spots on Radiography
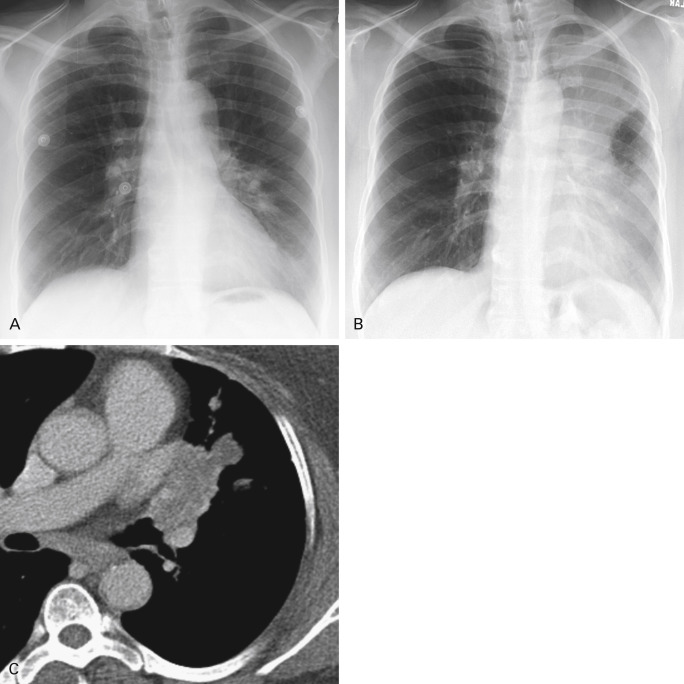
Chest radiographs can have blind spots, many of them due to overlapping anatomic structures such as skeletal bones. Specific regions such as the hila, where the pulmonary arteries and veins and the airways converge, may also be difficult to evaluate ( Fig. 21.6 ). Superimposed acute processes may mask findings of malignancy. Not infrequently, pneumonia can be the first indication of a central obstructing or partially obstructing lesion. Recurrent consolidations in the same location are highly suspicious ( Fig. 21.7 ). Consequently, radiographic follow-up to document resolution of pneumonia is advised. Chronic lung disease such as pulmonary fibrosis may also hinder identification of a focal abnormality. It is worth noting that preexisting chronic lung disease is a risk factor for lung cancer.
Characteristics of Lung Cancers Missed on Radiography
Retrospective studies of lung cancers not prospectively identified on chest radiography have demonstrated several common characteristics. In general, missed lung cancers were located in the upper lobes, although one study showed no difference in the location of missed and detected lung cancers. Another characteristic was small nodule size, on average 16 mm to 19 mm. An analysis of size differences in detection rate indicated that nodules 10 mm or smaller were missed 71% of the time. Nodules 10 mm to 30 mm in size were not detected 28% of the time. However, nodules 30 mm to 40 mm were missed at a rate of 12%, and nodules greater than 40 mm were identified 100% of the time. Centrally located nodules that were missed had a larger circumference than overlooked peripheral nodules. Overlying anatomic structures contributed to difficulty in detecting lung cancers. Many of the missed cancers had ill-defined borders and relatively low levels of density. A high pretest probability of lung cancer has been shown to promote detection rates, and in two studies, underdiagnosis of lung cancers in women appeared to be related to lower clinical suspicion.
Use of Special Radiographic Views
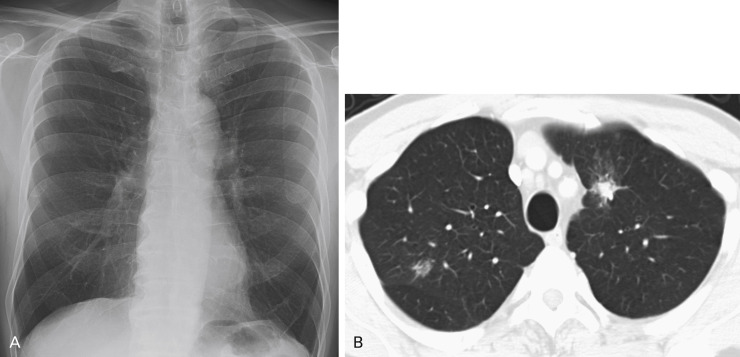
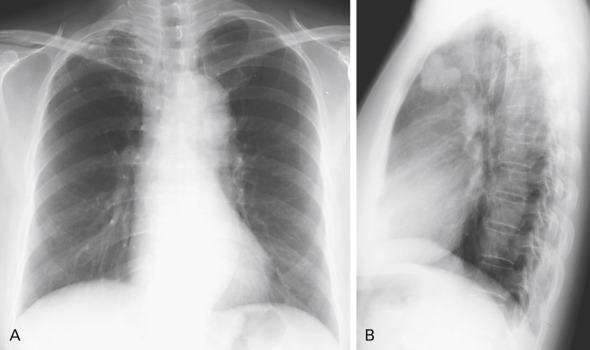
Disagreement exists over the importance of lateral projection in making a diagnosis of lung cancer. The authors of several studies have discovered a few cancers that could be visualized only on the lateral radiograph; although this represents a small number of cases, there is support for continued acquisition of the lateral chest radiograph in conjunction with the posteroanterior view ( Fig. 21.8 ). Oblique radiographs may also be helpful in determining whether an abnormality seen on standard views is external to the patient.
New Technology in Radiography
Technical advances in chest radiography have been made possible by digital radiographic technology.
Computer-Aided Detection
Computer-aided detection of pulmonary nodules on chest radiographs has been subject to the limitation of a high false-positive rate, but it does provide a so-called second reader effect by supporting the reading radiologist. It has been shown that double reads improve interpretation accuracy; however, in the current health-care system, practical reasons preclude having two trained radiologists read the same film. The use of computer-aided detection software systems has been shown to significantly enhance the accuracy and sensitivity of even experienced radiologists in detecting lung cancers on radiographs. In one study, the computer-aided detection program was able to identify 40.4% of subtle or very subtle cancers.
Dual-Energy Subtraction Radiography
Dual-energy subtraction technology uses two exposures of the frontal view of the chest obtained milliseconds apart at two different energy levels, or kVp. A postprocessing algorithm uses these exposures to virtually subtract the osseous structures from the radiograph, producing a soft tissue, thus providing a predominant image with greater conspicuity of lesions that are surrounded by air in the lungs, and fewer blind spots from overlapping skeletal structures ( Fig. 21.9 ). Nevertheless, persistent limitations exist, including suboptimal evaluation of the retrocardiac space, the periphery of the lungs, and the retrosternal space.
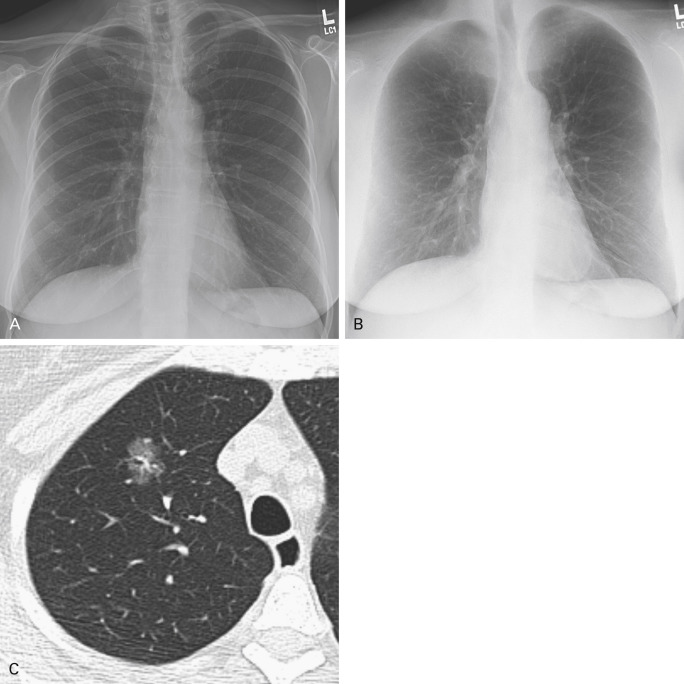
Early phantom studies showed improved detection of thoracic abnormalities with dual-energy subtraction technology, and observer studies have shown increased accuracy of lung nodule identification by both trained radiologists and residents. Although dual-energy subtraction technology has improved the detectability of all lung cancers, it is particularly helpful in identifying PSNs, which are the most likely to be malignant. Dual-energy subtraction technology can be used concurrently with computer-aided detection, but this combination has not yet been rigorously investigated.
Imaging with CT
The discovery of a nodule or persistence of a lung opacity on radiography can be an indication for CT evaluation. In a subset of the population of smokers and former smokers, screening for lung cancer with low-dose multidetector CT has been shown by the National Lung Cancer Screening Trial to decrease lung cancer mortality. In addition, lung nodules may be found incidentally during CT imaging for other reasons, such as motor vehicle trauma or pulmonary embolism evaluation in the emergency department.
Chest CT is much more sensitive than radiography for detecting lung nodules and more useful for characterizing abnormalities. CT allows for more accurate description of size and density of a lesion and can identify satellite lesions below the resolution of radiography. CT permits a more detailed inspection of the pleura, the mediastinum, the thoracic lymph node basins, and extrathoracic regions, including the liver and adrenal glands, both common sites of metastases from lung cancer.
Blind Spots on CT
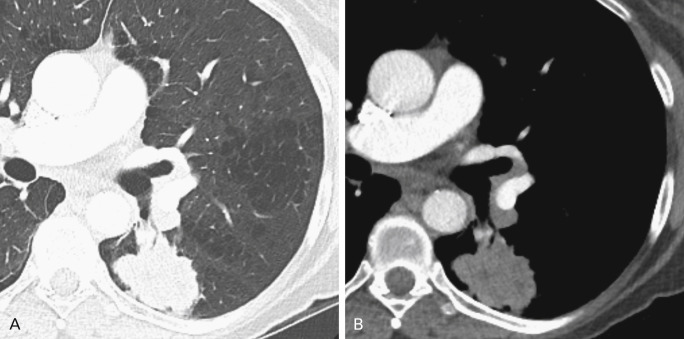
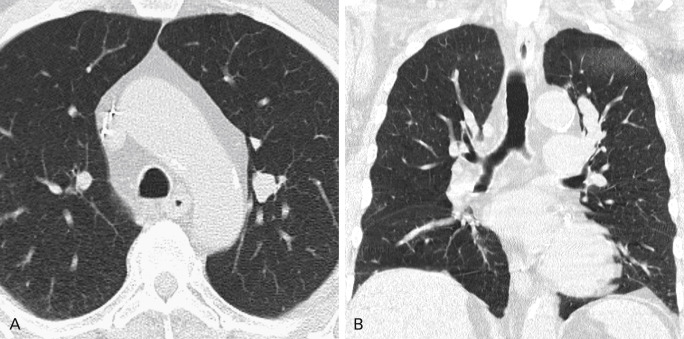
Several factors influence the recognition and diagnosis of lung cancer on CT. Small endobronchial lesions may be very subtle and difficult to see, necessitating meticulous examination of the airways. Small pulmonary nodules are missed on CT up to 47% of the time, usually because of central and/or peribronchovascular location ( Fig. 21.10 ). Adjacent airspace disease, including pneumonia or atelectasis, can obscure findings of malignancy. However, the Golden S sign denoting a central obstructing tumor with peripheral lobar collapse (classically, the right upper lobe) is highly suggestive of malignancy. On contrast-enhanced CT scans, differential enhancement of atelectasis compared with tumor or infection can help identify underlying lung lesions. The presence of intravenous contrast material also facilitates recognition of lymphadenopathy, particularly in the hilar regions, and of pleural involvement. The presence of other major findings on chest imaging can distract the reader and may hinder identification of a small malignancy.
Characteristics of Lung Cancers Missed on CT
The characteristics of lung cancers not detected on CT have been reviewed in several studies. The diameters of cancerous lesions missed on chest CT examinations are smaller than lesions missed on radiographs; the mean diameter in one study was 12 mm and in another was 9.8 mm for detection errors and 15.9 mm for interpretation errors. A large proportion of the missed tumors were subtle GGOs correlating mostly with well-differentiated lung adenocarcinomas. Central endobronchial lesions were most commonly missed in one study. Location in the lower lobes and in the perihilar regions contributed to detection failure, as did preexisting lung disease. A number of cancers were not prospectively identified in nonsmoking women. An acknowledged limitation in these studies is the thick 10-mm collimation and image reconstruction CT technique common to most of the examinations reviewed. A 5-mm collimation and image reconstruction algorithm is much more common on modern CT scanners, and even thinner collimation is standard in many academic centers.
New Technology in CT
Computer-Aided Detection
Computer-aided detection programs have also been developed to identify pulmonary nodules on CT ( Fig. 21.11 ). Studies using computer-aided detection as a second reader have shown improved sensitivity in nodule detection. False-positive results obtained using the software application are usually caused by vessels en face, vascular branch points, and/or artifacts. Although use of computer-aided detection to identify ground-glass nodules is more complicated than for solid nodules, some studies have shown that computer-aided detection also improves detection of ground-glass and mixed-attenuation nodules.

Maximum Intensity Projection
Maximum intensity projection images can facilitate the identification of small solid pulmonary nodules. The improvement in reader sensitivity is similar to that of computer-aided detection.
Use of CT in Staging of Lung Cancers
The revised version of the TNM staging system for NSCLC is discussed in detail in Chapter 25 . Adopted in 2009 after the evaluation of 67,725 cases of NSCLC, this edition of the TNM staging system categorizes TNM features into groups that best align with prognosis and treatment of the disease. This edition has also been recommended for staging of SCLC and bronchopulmonary carcinoid tumors. Chest CT is routine for baseline staging of lung cancer and for surgical planning, if the disease is potentially resectable. The study should include the adrenal glands in their entirety. As a result, much of the liver will also be within the field of view, although the entire liver is not typically imaged. Use of intravenous contrast material is preferred if not contraindicated by the patient’s allergy profile or by declining renal function. Multidetector CT acquires continuous volume datasets, which can then be used to create multiplanar reformatted images for additional information. Axial reconstructions are most optimal at 5 mm or less.
Tumor (T) Descriptors
Evaluation of lung tumors is first based on the size of the lesion, which correlates with prognosis. Smaller lesions have better outcomes: the 5-year survival for T1a lung carcinoma is 77%. By contrast, T4 lung cancer is associated with a 15% 5-year survival rate.
Accordingly, lesions up to 3 cm are T1, lesions up to 2 cm are T1a, and lesions greater than 2 cm up to 3 cm are T1b. The T2 category is divided into two parts: T2a lesions are greater than 3 cm to 5 cm, and T2b masses are 5 cm to 7 cm in size. T3 lesions are greater than 7 cm in diameter or located less than 2 cm from the carina. T4 tumors involve the carina directly.
Attendant factors upstage the T descriptor, including invasion of adjacent structures and collapse of the surrounding lung ( Fig. 21.12 ).
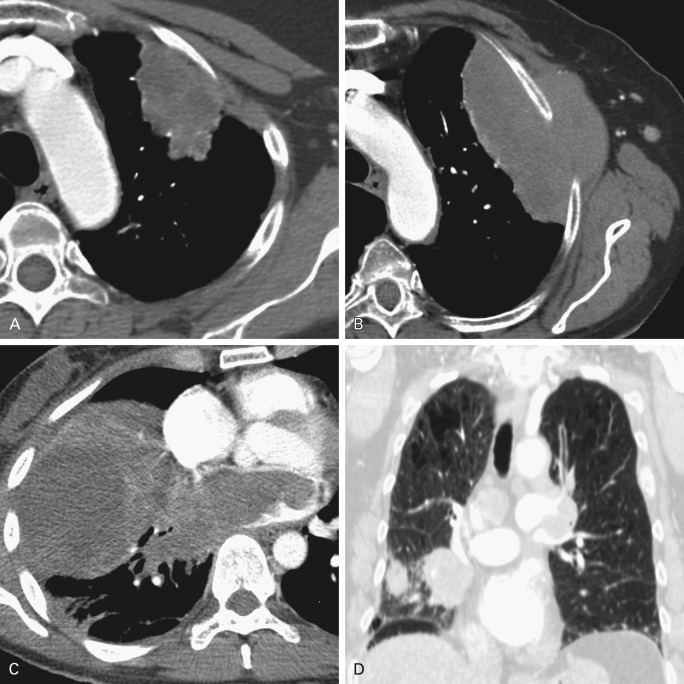
Direct Invasion of the Pleura
Tumors that abut a pleural surface, including the fissures, for a contact distance of greater than 3 cm, are considered suspicious for possible pleural invasion. Other findings that suggest invasion include eradication of the extrapleural fat plane, an obtuse angle between the tumor and pleural surface, and a tumor–pleura contact that exceeds the tumor height. Nevertheless, pleural invasion is not conclusively identified by imaging in most instances, excluding frank invasion of the chest wall; the pathologic evaluation of the resected specimen is definitive. Bone destruction and/or tumor tissue extending between the ribs is consistent with chest wall invasion, considered T3, which necessitates an en bloc resection of chest wall with the tumor.
Direct Invasion of the Mediastinum
Stranding of the mediastinal fat subjacent to the tumor, contact length greater than 3 cm along the mediastinal margin, and contact with the aorta of greater than 90° are suspicious for possible invasion of the mediastinum. Obliteration of the fat plane between the tumor and mediastinal structures is suspicious and also raises the possibility of mural invasion involving systemic and pulmonary vascular structures. Nevertheless, prediction of subtle mediastinal invasion with conventional imaging is low. Frank invasion of the heart or trachea can be seen and represents T4 disease.
Satellite Nodules
The presence of small lung nodules in addition to the dominant lesion also upstages the tumor. Satellite nodules within the same lung lobe as the primary malignancy constitute T3 disease. Nodules ipsilateral to the tumor but in a different lung lobe represent T4 disease. If the nodules are in the contralateral lung, they are considered metastatic, M1a.
Postobstructive Collapse or Pneumonitis
Lobar collapse around a central obstructing lesion constitutes T2 disease.
Lymph Node (N) Descriptors
Involvement of lymph nodes by tumor also has an effect on prognosis. Nodal basins commonly affected by lung cancer include the supraclavicular chains, the compartments of the mediastinum, and the hilar regions. Nodal disease occasionally but infrequently can be seen in the internal mammary chains, the parietal spaces, or the axillary and retropectoral spaces.
Lymph nodes that measure 1 cm or more in short-axis diameter on CT are considered to be suspicious for nodal metastatic disease despite relatively low sensitivity and specificity. However, not all metastatic lymph nodes meet the size criteria for enlargement and not all enlarged nodes are metastatic. An analysis of resected nodes measuring 2 cm to 3 cm found that 37% had no metastatic involvement. When nodal disease is not identified on standard CT, 18 F-2-deoxy- d -glucose (FDG)-PET or PET–CT can be useful for identifying small malignant nodes as well as for guiding biopsies. Lymph node sampling is performed to confirm the presence of nodal disease based on suspicious CT or PET–CT findings.
Absence of nodal metastatic disease is considered N0. The presence of hilar or intrapulmonary lymphadenopathy ipsilateral to the tumor constitutes N1 disease. Metastatic adenopathy in the ipsilateral mediastinum and/or subcarinal region qualifies as N2 disease. The spread of metastases to the contralateral mediastinum, the contralateral hilum, or the supraclavicular or scalene nodes of either side denotes N3 disease and raises the stage to IIIB, which is considered unresectable.
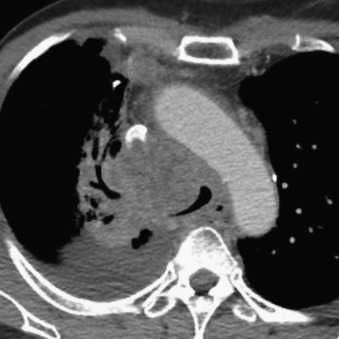
When extensive bulky nodal disease is present, compression and/or frank invasion of the mediastinal vascular structures is possible, with danger of superior vena cava syndrome. Tracheal and/or esophageal compromise is also a concern ( Fig. 21.13 ).