Postmastectomy radiation therapy (PMRT) can improve survival and locoregional control in patients with invasive breast cancer. The optimal timing and technique of breast reconstruction in patients requiring PMRT is controversial. The purpose of this chapter is to examine the most recent literature on breast reconstruction in patients receiving PMRT to help breast reconstructive surgeons make the best treatment decisions.
With an increasing number of patients receiving PMRT, the decision of whether to offer implant-based or autologous tissue breast reconstruction has never been so relevant. Recent studies indicate that implant-based breast reconstruction in patients receiving PMRT is problematic.
Studies evaluating the outcomes of 2-stage breast reconstruction, with placement of a tissue expander followed by placement of a permanent breast implant after PMRT, consistently reveal high rates of acute and chronic complications and poor aesthetic outcomes.1 Capsular contracture that results from PMRT not only distorts the appearance of the breast, but also causes chronic chest wall pain and tightness that can be crippling. Many surgeons attribute the poor outcomes with implant-based breast reconstruction to older, less precise techniques of radiation delivery. However, even with modern radiation delivery techniques, complication rates with implant-based reconstruction are high.
Ascherman and colleagues2 recently evaluated the complications and aesthetic outcomes of 104 patients who underwent 2-stage implant-based reconstruction. Twenty-seven patients also underwent radiation therapy, either before mastectomy (patients who were undergoing salvage mastectomy after lumpectomy and radiation therapy) or after mastectomy. In all 27 of these patients, radiation therapy was completed before the tissue expander was exchanged for a permanent breast implant or before the expander port was removed. Despite use of the latest prosthetic materials and modern radiation delivery techniques, the overall complication rates for the irradiated and nonirradiated breasts were 40.7% and 16.7%, respectively (p ≤ 0.01). Complications that resulted in removal or replacement of the prosthesis occurred in 18.5% of the irradiated and only 4.2% of the nonirradiated breasts (p ≤ 0.025). In addition, the extrusion rate was higher for implants in irradiated breasts (14.8% vs 0%; p ≤ 0.001). Breast symmetry scores were significantly higher in the patients who did not receive radiation therapy (p < 0.01). Despite the retrospective study design, these findings are important because they represent the experience of a single surgeon who evaluated patients treated using the latest prosthetic devices with total submuscular coverage and modern radiation therapy techniques.
In another recent study, Benediktsson and Perbeck3 used applanation tonometry to prospectively evaluate rates of capsular contracture around saline-filled, textured implants in 107 patients who underwent mastectomy with immediate breast reconstruction. Twenty-four of the patients received PMRT. Radiation was delivered using a modern, 3-beam technique with a combination of photons and electrons, and reconstruction was accomplished using the latest prosthetic devices. The rate of capsular contracture was significantly higher for irradiated breasts than for nonirradiated breasts (41.7% vs 14.5%; p = 0.01). The difference in contracture rates was not evident during the first 6 months, but was highly significant thereafter, even 5 years later.
In 2006, Behranwala and colleagues4 published results from a series of 136 breast reconstructions with a median follow-up of 4 years: 62 reconstructions were performed with submuscular implants alone and 74 with a latissimus dorsi flap plus an implant. Forty-four reconstructed breasts received PMRT. Capsule formation was detected in 14.1% of the nonirradiated reconstructed breasts and 38.6% of the irradiated reconstructed breasts. On univariate analysis, PMRT was the only variable related to capsule formation (p < 0.001). Significant differences in geometric measurements of the breasts and worse photographic assessments were seen in the group that received PMRT. Pain that persisted for 2 years or more after surgery was present in 27% of breasts with capsular contracture and less than 1% of breasts without capsular contracture.
Impact of Performing the Tissue Expander–Permanent Implant Exchange before Rather Than after Radiation Therapy
In a departure from the standard approach of exchanging the tissue expander for the permanent breast implant after PMRT, Cordeiro and colleagues5 have been exchanging the expander for the implant before PMRT. Whereas Ascherman and colleagues2 had to remove or replace the implant in 18.5% of the irradiated patients who had the permanent implant placed after PMRT, Cordeiro and colleagues5 had to remove or replace the implant in only 11.1% of 81 patients. In addition, their technique resulted in acceptable aesthetic outcomes in most patients.
Although placing the permanent implant before PMRT should decrease the occurrence of acute wound healing problems (ie, wound dehiscence, infection, and implant exposure), there is no known pathophysiologic explanation for the relatively low frequency (5.9%) of severe (Baker classification, grade 4) capsular contracture described in Cordeiro and colleagues’ study. In fact, the literature is replete with studies showing much higher rates of severe capsular contracture—in the range of 16% to 68%—when the permanent breast implant is in situ during the delivery of PMRT.3,4
Another consideration with the approach of Cordeiro and colleagues5 is that it is not generally feasible in patients who undergo neoadjuvant chemotherapy, in whom PMRT is usually started within 4 weeks after mastectomy, leaving inadequate time for postoperative expansion and exchange of the expander for the permanent implant. This issue is important because many medical centers are increasing their use of neoadjuvant chemotherapy in patients with locally advanced breast cancer, even in patients with lesser degrees of local and regional disease, and many of the patients who are either known or suspected preoperatively to require PMRT are the same patients who may benefit from neoadjuvant chemotherapy.
In another, more recent study from the Memorial Sloan-Kettering group,6 the authors further evaluated their approach of exchanging the expander for the implant before PMRT in 12 patients (only 10 patients were available for follow-up) who underwent bilateral mastectomy and required PMRT in only 1 of the reconstructed breasts. When the authors compared the degree of capsular contracture (Baker classification) between the irradiated and contralateral nonirradiated breast, they found no difference in 4 patients, a single-grade difference in 5 patients, and a 2-grade difference in 1 patient. This series was too small and the median follow-up period (23.5 months) too short to permit any significant conclusions, especially considering that capsular contracture sometimes does not occur until 5 or more years3 after reconstruction and that it may progress over time. However, a statistically significant comparative analysis should be possible as the authors’ experience increases and more patients with longer follow-up are available for study. Another potential concern with this study is the authors’ suggestion that the contralateral breast was an ideal control. In patients with bilateral implant-based reconstruction, the contralateral breast often receives radiation scatter in its medial aspects because of treatment of the medial aspect of the involved breast and/or the internal mammary nodes.7 Therefore, in a patient who undergoes bilateral implant-based breast reconstruction and PMRT of 1 breast, the contralateral breast may also be at increased risk for capsular contracture.
Current Role of Reconstruction with a Latissimus Dorsi Flap Plus a Breast Implant in Breast Cancer Patients Who Receive PMRT
In a study reported by Evans and colleagues8 that examined the outcomes of patients who underwent breast reconstruction with an implant and an autologous tissue flap, the authors found that the addition of the tissue flap—either a transverse rectus abdominis myocutaneous (TRAM) flap or a latissimus dorsi myocutaneous flap—did not appear to offer protection against capsular contracture, a common complication of PMRT. The authors advocated breast reconstruction with autologous tissue alone in patients who have undergone or are about to undergo PMRT.
In 2007, Spear and colleagues9 published a retrospective review of the role of the latissimus dorsi flap with an implant in reconstruction of previously irradiated breasts. The authors concluded that in patients with unsatisfactory outcomes after 2-stage implant-based reconstruction as a result of the adverse effects of PMRT, the breast contour can be improved by adding a latissimus dorsi flap, generally to the inferior pole of the breast.
Immediate Implant-Based Breast Reconstruction Can Compromise the Design of the Radiation Treatment Fields
Not only can PMRT adversely affect the aesthetic outcome of immediate implant-based breast reconstruction, but there is increasing evidence that such reconstructions may interfere with the delivery of PMRT (Fig. 75-1).7,10-16 This interference can occur with a breast implant, a fully inflated tissue expander in situ on the chest wall, or even a partially deflated expander. The sloping contour of a reconstructed breast results in an imprecise geometric match of the medial and lateral radiation fields, which can lead to underdosing of the chest wall, especially centrally underneath the breast prosthesis and near the internal mammary nodes.15 The sloping contour also leads to nonuniform thickness of the chest wall across the width of the electron beam field, which can lead to a nonhomogeneous dose within the treatment field because the electron beam dose falls off as a function of tissue thickness.15 Chest wall treatment in patients who have undergone reconstruction must be accomplished using traditional, 2-beam tangential fields alone rather than modern, 3-beam technique.7,15,16 Unfortunately, it is not feasible to reconstruct the medial and apical breast to result in more favorable geometry for radiation treatment because of the higher density of flap tissues from the abdomen and gluteal regions.
Figure 75-1
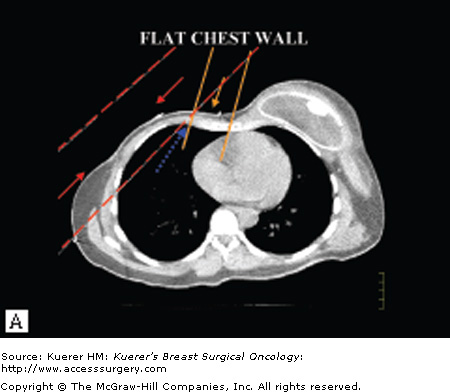
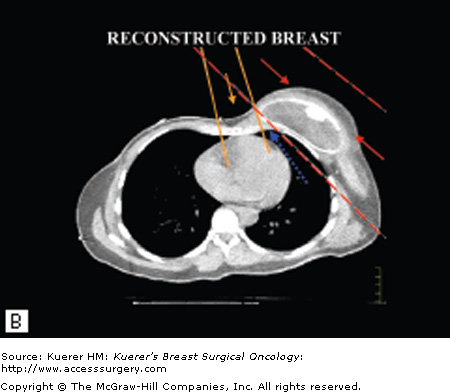
Potential problems with radiation delivery after breast reconstruction. A. Typical approach for chest wall irradiation. The medial chest wall and internal mammary nodes are treated with an anterior electron beam field that is geometrically matched to 2 lateral chest wall electron beams. The medial chest wall beam has a rapid dose fall-off after the chest wall and lung interface (orange arrow) and when combined with the lateral photon fields (red arrows) treats a small volume of lung. The flat chest wall surface allows for a relatively precise junction of the fields (blue arrow). B. The sloping contour of a reconstructed breast leads to an imprecise geometric match of the medial and lateral radiation fields, which can lead to underdosing of areas of the chest wall (blue arrow) and a nonuniform dose distribution (red arrows). A second consequence of the sloping contour is that the thickness of the chest wall across the width of the electron beam field becomes nonuniform. The electron beam field falls off as a function of tissue thickness, so that nonuniformity can also lead to an inhomogeneous dose distribution within the treatment field. (Reproduced with permission from Buchholz TA, Strom EA, Perkins GH, et al. Controversies regarding the use of radiation after mastectomy in breast cancer. Oncologist. 2002;7:539-546.)
Although recent studies17,18 have found that the metallic port within a tissue expander does not result in significant scatter, which could lead to “hot” or “cold” spots that alter the homogenous treatment of the chest wall, the problem of the beam geometry and whether it allows treatment of all necessary tissue regions without increased damage to the heart or lungs remains a concern. On the other hand, some radiation oncologists believe that the effect of a breast implant or inflated tissue expander is negligible.19
In 2005, Schechter and colleagues7 at the University of Texas MD Anderson Cancer Center found that immediate breast reconstructions may limit treatment planning for PMRT. They retrospectively reviewed the records of 152 patients treated with PMRT, 17 of whom underwent immediate breast reconstruction and had expanders, flaps, and/or implants in place at the time of PMRT. The authors evaluated the impact of various reconstructive techniques on the ability to treat the breadth of the chest wall, treat the internal mammary nodes within the first 3 interspaces, avoid the lung, and avoid the heart (Table 75-1). They found that completely deflated expanders resulted in no compromise, a partially deflated expander prevented treatment of the internal mammary nodes, and fully inflated expanders moderately or severely compromised treatment of the internal mammary nodes and chest wall.
| Laterality | Immediate Reconstruction Technique | Postmastectomy Irradiation Technique | Avoidance of Heart | Avoidance of Lung | Treatment of Ipsilateral Internal Mammary Region | Treatment of Chest Wall Breadth | Comments |
|---|---|---|---|---|---|---|---|
| R | TRAM | TANGENTS | 1 | 1 | 0 | ½ | |
| R | TRAM | TANGENTS | 1 | 1 | 0 | ½ | |
| R | TRAM | TANGENTS | 1 | 1 | 0 | ½ | Bilateral reconstruction |
| R | TRAM | TANGENTS | 1 | 1 | 0 | ½ | Bilateral reconstruction |
| R | EXPANDER FI | TANGENTS | 1 | 1 | 0 | ½ | |
| R | EXPANDER PD | TANGENTS | 1 | 1 | 0 | 1 | |
| R | EXPANDER CD | TANGENTS + IMC e– | 1 | 1 | 1 | 1 | |
| L | LATISSIMUS (Dbl) | TANGENTS | 1 | 1 | 0 | ½ | Bilateral reconstruction |
| L | LATISS + SALINE | TANGENTS | 1 | 1 | 0 | 0 | |
| L | TRAM | PD TANGENTS | 1 | ½ | ½ | ½ | |
| L | TRAM | PD TANGENTS | ½ | ½ | 1 | 1 | |
| L | TRAM | PD TANGENTS | 1 | ½ | 1 | ½ | |
| L | TRAM | PD TANGENTS | 1 | ½ | 1 | 1 | |
| L | TRAM | PD TANGENTS | 1 | 1 | 0 | 0 | |
| L | EXPANDER FI | PD TANGENTS | 1 | 1 | ½ | ½ | Bilateral reconstruction |
| L | LATISS + EXP CD | TANGENTS + IMC e− | 1 | 1 | 1 | 1 | |
| L | TRAM | TANGENTS + IMC e− | 1 | 1 | 1 | 1 | |
| L | TRAM | TANGENTS + IMC e− | 1 | 1 | 1 | 1 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree