aComplication rates increased with the extent of hepatectomy (p < 0.05)
Columns divided by magnitude of hepatectomy; DVT, deep venous thrombosis; PE, pulmonary embolus; OR, operating room.
Three complications specific to liver surgery are bleeding (intraoperative and early postoperative), bile leak (and associated organ space infection), and postoperative hepatic insufficiency (PHI, or liver failure) with liver-related mortality. Generally, PHI can be split into “early” and “late” phases, in which the late phase often leads to an irreversible cascade toward liver-related mortality that can happen well past 30 postoperative days. Late PHI mandates that surgeons look beyond the typical postoperative interval of 30 days to completely capture all liver-related morbidity and mortality. In fact, up to one-third of posthepatectomy deaths occur between 30 and 90 postoperative days.
Predictors of major complications can be divided into three categories—patient comorbidities, biochemical abnormalities, and perioperative risk factors (including magnitude of operation). These factors generally include the American Society of Anesthesiologists (ASA) class, smoking, elevated alkaline phosphatase, low albumin, elevated partial thromboplastin time (PTT), extent of hepatectomy, intraoperative or postoperative transfusions, and prolonged operative time. The last three factors are closely related, and thus surgeons should be cautious about pairing major simultaneous operations with major hepatectomies. While not all risk factors are completely reversible, certainly many are potentially modifiable through preoperative optimization of comorbidities and choosing operations of lesser extent when oncologically feasible.
Besides the typically reported surgical quality outcomes of postoperative complications and death, surgeons should also understand and further study the secondary implications of major morbidity—failure to return to preoperative performance status and failure to achieve intended oncologic therapy. The long-term benefits of the most extraordinary and aggressive hepatectomy may be completely negated if the patient loses his or her independence and becomes bound to a nursing home, is unable to return to his or her previous functional state, or is unable to complete his or her planned adjuvant cancer therapy.
The goal of this chapter is to describe the diagnosis, management, and risk factors for three major liver-related complications specifically associated with hepatic resection.
INTRAOPERATIVE AND POSTOPERATIVE HEMORRHAGE
Definition
Historically, hemorrhage has been the most feared complication of hepatectomy. “Bleeding” broadly encompasses a range of complications including catastrophic intraoperative hemorrhage, intraoperative bleeding requiring intraoperative and recovery room transfusions, postoperative hemorrhage requiring transfusions or even return to the operating room, symptomatic anemia requiring postoperative transfusions, or small drops in hemoglobin with no clinical sequelae.
Based on retrospective institutional reports, the posthepatectomy hemorrhage (PHH) rate ranges from 1% to 8%. Because of its broad definition, the International Study Group of Liver Surgery (ISGLS) attempted to define PHH based on transfusion requirement and clinical severity. The proposed definition is a hemoglobin drop greater than 3 g/dL after completion of surgery and/or transfusion for any postoperative hemoglobin drop and/or invasive intervention (interventional radiology [IR] or surgery) to stop bleeding (Table 18.2). Detection of hemorrhage can be via frank blood loss apparent in perihepatic drains (drain fluid hemoglobin >3 g/dL), examination consistent with intra-abdominal hematoma, or active bleeding with any radiographic diagnosis. The ISGLS definition of PHH is specific to the postoperative period and does not include patients who receive up to 2 units packed red blood cells (PRBC) immediately after surgery in the recovery room. Functionally, PHH is split into three grades, A to C. Grade A PHH is defined as transfusion of ≤2 units PRBC with no significant change in clinical management. Grade B PHH corresponds to transfusion of greater than 2 units PRBC, often with changes in management including further radiographic evaluation. Bleeding that requires any invasive intervention is categorized as grade C. Importantly, grades B and C PHH can be associated with mortality rates as high as 17% and 50%, respectively.
TABLE 18.2 International Study Group on Liver Surgery Definitions of Posthepatectomy Complications
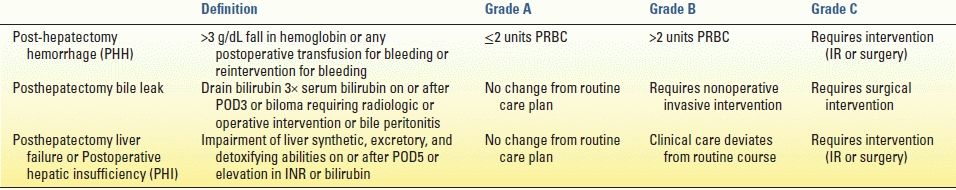
PRBC, packed red blood cells; IR, interventional radiology; POD, postoperative day; INR, international normalized ratio.
The ACS NSQIP defines major postoperative transfusion as administration of greater than 4 units PRBC after leaving the operating room. Currently, the national rates of any intraoperative transfusion, greater than 4 units intraoperative PRBC transfusion, and major postoperative transfusion greater than 4 units PRBC are 26.4%, 9.0%, and 0.8%, respectively. This nationwide sample suggests that a significant intraoperative use of blood products exists, but the major PHH seems to be rare.
Diagnosis and Management
The diagnosis of bleeding or hemorrhage involves clinical evaluation supplemented by laboratory data, drain output (if available), and radiographic imaging when indicated. Clinically, the patient may first develop tachycardia (unless beta-blocked) and/or oliguria, with hypotension and diaphoresis only coming later as worsening hypovolemic shock ensues. As it is atypical for a postoperative liver surgery patient to need a large volume of intravenous fluid, if a patient has required several fluid boluses in the early postoperative period, a necessary workup into reasons for hypotension or low urine output is required. While ruling out extrinsic causes (excessive narcotics or epidural anesthesia) and cardiovascular events, bleeding should be high in the differential. As bleeding becomes more likely, the question turns to clinical significance.
The abdominal examination may not be helpful as most patients will have incisional tenderness. The exception is with massive hemoperitoneum that should be readily apparent and warrants urgent measures. A change in the character of the abdominal drain fluid to a more sanguineous consistency may be a sign of bleeding that can be confirmed with serum and drain hemoglobin measurement. While the ISGLS PHH definition includes drain hemoglobin of greater than 3 g/dL, in many cases when active bleeding is apparent, no fluid sample is needed to secure the diagnosis. The criteria for transfusion depend on the severity of anemia (serum hemoglobin <7 g/dL or relative drop that is hemodynamically relevant), the degree of coagulopathy, and the patient’s overall condition. Given the evidence that transfusions may be associated with postoperative complications, which may in turn be detrimental to oncologic outcome, routine transfusions should be discouraged unless warranted by hemodynamic compromise with end-organ dysfunction. A contained hematoma does not require immediate intervention if tamponade stops further bleeding. In a stable patient with clinical evidence of bleeding, radiographic imaging with angiography (either by IR angiogram or by computed tomography [CT], angiogram) may allow for diagnosis and therapeutic intervention. However, if the patient is unstable or if IR cannot arrest the bleeding, then a return to the operating room is necessary. Laboratory values including platelet count, prothrombin time (PT), PTT, and fibrinogen help guide the resuscitation and replacement of deficient coagulation factors.
Preoperative and Intraoperative Considerations
Preoperative risk factors for major postoperative transfusion needs (defined by ACS NSQIP as >4 units PRBC) include preoperative bleeding disorder, ASA class ≥3, low albumin, and preoperative anemia. Right and extended hepatectomies are also at greater risk for PHH. One of the key reasons mortality from hemorrhage is now less common is better understanding of hepatic vascular anatomy and more advanced parenchymal transection techniques, either with or without inflow control. The numerous methods of parenchymal dissection and transection are discussed in Chapter 13. A few salient technical issues are reviewed here. Although the general use of stapling devices for parenchymal transection may be associated with higher bleeding rates, the targeted use of endovascular staplers to control vascular pedicles may increase the speed and safety of liver surgery. Newer tissue dissectors, including ultrasonic and water-jet devices, facilitate precise parenchymal dissection to identify vascular and biliary structures, which can be clipped or tied precisely rather than blindly transected without ligation. Tissue-sealing devices, borrowed from minimally invasive surgery, also aid in coagulation of the parenchymal before division.
Good planning and communication with the anesthesia team before and during surgery facilitate the safety of hepatectomy. During the time-out process, a discussion among the surgeon, nursing staff, and anesthesia team, of the extent of hepatectomy, plan for inflow clamping (Pringle maneuver), predicted blood loss, and blood product availability and antibody status, can decrease intraoperative surprises.
The concept of “low central venous pressure (CVP) anesthesia” is recommended for liver resections. This concept is critical to reducing intraoperative hemorrhagic events. However, accurate monitoring of CVP does not require a central venous catheter. Regardless of the method of intravascular volume monitoring, intravenous fluid administration should remain low (the surgeon can aid in monitoring this by examining the inferior vena cava) until the transection is completed. Keeping the CVP low significantly reduces venous bleeding during the parenchymal transection. Laparoscopic liver surgery has been associated with decreased blood loss because the required pneumoperitoneum decreases venous oozing from the parenchymal surface. The use of stay stitches to elevate the liver out of the abdominal cavity (with laparotomy pads behind the mobilized liver) may elongate and compress hepatic veins, decreasing venous bleeding during transection.
Regardless of the parenchymal dissection and transection technique, after the resection, the liver edge should be carefully inspected for vessels or bile ducts that were not adequately ligated. A gentle Valsalva maneuver by the anesthesia team can expose hepatic vein tributary bleeding, which may require suture ligation. The role of topical hemostasis to the liver transection bed with cautery, coagulant sheets, or fibrin glue is frequently debated. With no overt bleeding sites, some surgeons leave the surface alone, arguing that a viable liver edge may help decrease postoperative bile leak. Others cauterize the entire cut surface with or without placement of a hemostatic agent (fibrin glue or cellulose netting/gauze) applied to the surface. Others take the time to develop and place an omental flap to cover the raw liver surface.
Although the elements that contribute to major bleeding events in liver surgery are complex, an operative strategy that identifies and controls major hepatic vein tributaries and portal triad branches before and during the parenchymal dissection is the best way to avert major bleeding (and not coincidentally bile leak as well). Such a strategy is facilitated by careful preoperative planning that includes a thorough understanding of the patient’s liver anatomy including the relationship of the liver vasculature to the target lesion(s). High-quality three-phase liver protocol CT scan or high-resolution magnetic resonance imaging (MRI) is important. General abdominal CT scans (such as those ordered during emergency room evaluation for abdominal pain) are inadequate and may be dangerous if used as a guide. Intraoperative ultrasound is also essential to permit exact dissection along the correct planes. This precise dissection balances adequate oncologic margins with maximal parenchymal sparing when possible, dividing only the necessary vessels and biliary pedicles. While bleeding risk cannot be fully eliminated, with adequate understanding of the patient’s liver anatomy, good communication with the entire operating room team, and proper use of available intraoperative surgical technology, the risk of intraoperative and postoperative hemorrhage can be purposefully reduced to minimal rates.
BILE LEAK AND ORGAN SPACE INFECTIONS
Definition
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree