Fig. 10.1
A cat with squamous cell carcinoma. Ulcerated, crusting lesions on the planum nasale and pinna in regions of nonpigmented fur and skin (Image provided by Michael Kent, DVM)
Multicentric squamous cell carcinoma in situ (Bowen’s-like disease) is seen in cats and has been described in the dog [12, 13]. In contrast to invasive squamous cell carcinoma in cats, exposure is not related to ultraviolet light exposure and lesions frequently occur in haired, pigmented skin.
Melanocytic Tumors
Melanocytic tumors are common in dogs and horses. Gray horses are extremely prone to development of melanocytic tumors. In dogs, location of the tumor is of critical importance to the malignant potential of these tumors. Generally, tumors arising from the haired skin are benign, whereas those arising from mucocutaneous junctions are malignant. Benign cutaneous melanomas typically show little nuclear or cellular pleomorphism and have fewer than three mitotic figures per ten high-power fields (HPF). The majority of cases of malignant melanoma in dogs occur at mucocutaneous junctions or in the oral cavity.
Round Cell Tumors
Cutaneous lymphoma is seen in dogs and cats. The clinical appearance of cutaneous lymphoma shows marked variability and may manifest as patches, plaques, or tumors. Similar to cutaneous lymphoma in humans, cases in dogs and cats are typically classified as epitheliotropic and nonepitheliotropic. In epitheliotropic lymphomas, T-cells have an affinity for epidermis and adnexal epithelium. Nonepitheliotropic lymphomas are B- or T-cell origin and have clusters or sheets of neoplastic lymphocytes. Cutaneous lymphoma tends to be progressive and multicentric and will progress to involve regional lymph nodes and viscera (Fig. 10.2).
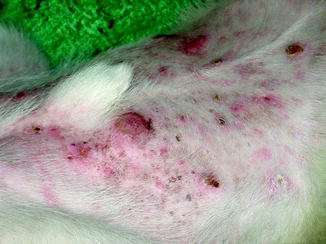

Fig. 10.2
Multifocal skin lesions over the abdomen of a dog with cutaneous lymphoma (Image provided by Michael Kent, DVM)
Other cutaneous round cell tumors are seen with varying frequency in animals including several tumors seen commonly in animals that are not seen frequently in humans. Although multiple myeloma in animals can have cutaneous involvement, cutaneous plasma cell tumors are usually de novo proliferations that are not associated with primary bone marrow disease. Most cutaneous plasmacytomas in dogs are benign and cured by surgical excision. Cutaneous mast cell tumors are among the most common cutaneous neoplasms seen in dogs and cats and can be focal or multicentric. There are species variations in the biologic behaviors of mast cell tumors, but tumors of mast cells are very rare in humans. Cutaneous histiocytomas are unique to dogs and are benign spontaneously regressing tumors. Its malignant counterpart, the histiocytic sarcoma, is seen in dogs but rarely described in humans.
Comparative Clinical and Pathologic Aspects of Specific Animal Skin Cancers
Melanoma
Melanoma is a common and malignant disease seen in human and veterinary medicine. Histopathology is the most common means of diagnosis although the disease has a highly variable appearance histologically that can complicate diagnosis. Aspects of the histologic appearance of a lesion are often used to predict the biologic behavior of the tumor. Melanocytic tumors in humans and animals have important clinical and pathologic similarities and differences.
Clinical Aspects of Melanoma
Clinically, melanoma is reported to account for 3 % of all neoplasms and 7 % of all malignant tumors in dogs [14]. The common sites for melanoma in dogs are quite different from humans where the vast majority are cutaneous. The most frequently affected sites in dogs are the oral cavity (56 %), lip (23 %), skin (11 %), and digit (8 %) [12, 15]. Oral melanoma is the most common oral tumor in dogs and the vast majority are malignant (Fig. 10.3). Dogs with oral melanoma may present when a mass is observed but more commonly present for signs of dysphagia, halitosis, ptyalism, or bleeding [15, 16]. The most common metastatic sites for oral melanoma to spread to are the regional lymph nodes, lung, and viscera although melanoma metastases have been documented at nearly any site [17–20]. In dogs and humans, there is an increased frequency of oral melanoma in males compared to females [17, 21–24].
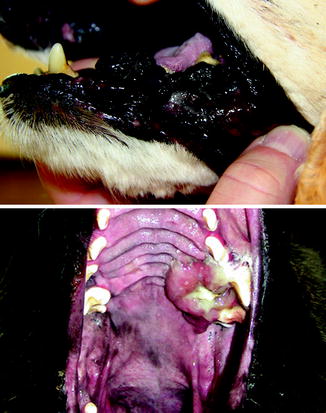

Fig. 10.3
Pigmented oral melanoma (top) and amelanotic oral melanoma in dogs (Image provided by Michael Kent, DVM)
Only approximately 5 % of cutaneous melanomas in dogs are malignant in notable contrast to humans [11, 12, 15]. Certain breeds of dog have an increased incidence of melanoma, usually dogs with darkly pigmented skin. Predisposed breeds include the Miniature Schnauzer, Scottish Terrier, and Standard Schnauzer [25, 26]. This is an interesting contrast to humans where cutaneous melanoma appears to be less common in individuals from races with darkly pigmented skin [27–29].
Cutaneous melanoma is common in horses. Over 90 % are benign at initial presentation, but the majority of these are believed to have the potential to undergo malignant transformation over time [15, 30–37]. Gray and white horses are markedly predisposed and develop melanoma with increasing prevalence as they age, often developing multifocal cutaneous sites of involvement [32, 38].
Melanoma is uncommon in cats, but they do develop oral, cutaneous, and ocular forms [39–44]. Over 50 % of melanomas in cats are malignant. Melanoma is also seen in many other domestic species of animal including cattle, sheep, goats, alpaca, and swine [45–48]. The Sinclair miniature breed of pig has a genetic predisposition to develop melanoma and has been used as a model for human spontaneous cutaneous melanoma [49–51].
In animals, the location of a melanoma lesion is an important prognostic indicator. Melanoma involving the oral cavity, mucocutaneous junctions, and the subungual region is considered malignant regardless of histologic features. In humans, melanomas involving the oral cavity or mucocutaneous junctions are also malignant.
Gross Pathology and Histopathology of Melanoma
Regardless of species, melanoma varies widely in gross appearance. Masses may be any color including gray, brown, black, or red [13, 15, 52]. Cutaneous melanoma can appear as a smooth dome, a nodule, a plaque, or a lobulated mass [53]. Cutaneous melanomas in horses are usually flat and firm although multiple lesions may coalesce creating a cobblestone appearance [32]. The skin over a lesion may be alopecic or ulcerated. Masses can be compressive but more commonly are infiltrative and unencapsulated. They may be poorly defined without forming a discrete mass and they may efface normal structures.
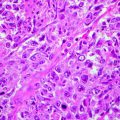
Histologically, cutaneous melanomas in animals are not differentiated the same way as human melanoma. The term “nevus” which is used to describe pigmented melanocytic lesions of the epidermis and dermis in humans is not used in veterinary pathology [15, 26]. Consequently, veterinary pathologists do not typically differentiate dysplastic nevi, from melanoma in situ, from early invasive melanoma. Most of the melanomas in animals are invasive masses in contrast to the thin, superficial, spreading type of melanoma that is seen most commonly in humans [15]. Human and canine melanoma can both be described by whether the neoplastic cells are spindle cells, epithelioid cells, round cells, or a mixture of these morphologies. Oral melanoma in dogs and humans is malignant and has a poor prognosis regardless of histologic type and morphology.
Poorly differentiated and amelanotic melanomas present a diagnostic challenge for the pathologist. A number of immunohistochemical methods have been developed to improve the diagnosis of melanoma although currently available markers are either not specific for melanoma or are only variably expressed by melanoma. Therefore, the diagnosis often depends on the expertise of the pathologist in combination with immunohistochemical findings. Nearly all melanomas are vimentin positive and cytokeratin negative in humans, dogs, and cats, but this is not specific for melanoma as many sarcomas have a similar pattern [44, 54–56]. S100 is a calcium-binding protein found in the cytoplasm and nucleus. Most melanomas are S100 positive, but this is also not specific for melanoma [44, 56, 57]. Similarly, neuron-specific enolase is used to help identify melanocytic tumors but is not specific [16, 58]. Melan-A is the most specific IHC marker for melanoma and is usually strongly positive in melanocyte cytoplasm [16]. One study of 122 canine oral melanomas showed that 92 % were Melan-A positive including a number of amelanotic tumors [16].
Squamous Cell Carcinoma
Clinical Aspects of Squamous Cell Carcinmoma
Squamous cell carcinoma is the most common malignant tumor of the skin in cats and second most common in dogs following mast cell tumors [59]. Due to its association with sun damage, incidence correlates to geography and climate.
Squamous cell carcinomas may present either as plaques, erosive lesions, or nodular masses of varying size [59]. Initial plaques will often progress to large erosive or nodular lesions (Fig. 10.4). Multiple lesions are common. The planum nasale is a common tumor site for cats with squamous cell carcinoma but is much less common in dogs. White-faced cats and short-coated dogs with light coat color have the highest incidence of squamous cell carcinoma [60, 61].
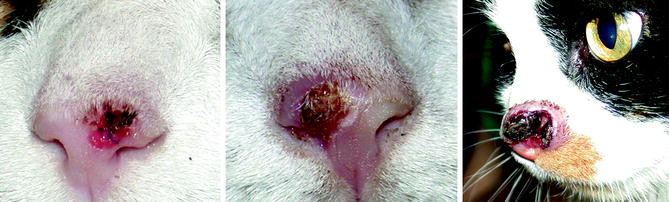

Fig. 10.4
From left to right, the planum nasale of three cats with progressively more invasive squamous cell carcinoma (Image provided by Michael Kent, DVM)
Gross Pathology and Histopathology of Squamous Cell Carcinoma
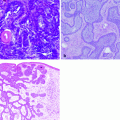
Most squamous cell carcinomas in dogs and cats are well differentiated, arising from superficial hair follicles without involvement of the epidermis [13]. The adjacent epidermis often has some degree of keratinocyte hyperplasia or dysplasia associated with solar damage. Poorly differentiated squamous cell carcinoma lesions tend to have high mitotic activity. For anaplastic lesions, immunohistochemistry to detect high molecular weight cytokeratins may be helpful in differentiating this tumor [59].
Lesions of multicentric squamous cell carcinoma in situ have irregular epidermal hyperplasia with marked hyperkeratosis and parakeratosis. They frequently have hyperpigmentation of the stratum corneum. There is often marked disruption of normal epithelial stratification. The differentiation between multicentric squamous cell carcinoma in situ and actinic keratosis is usually made clinically based on the location of lesions.
Basal Cell Carcinoma
Clinical Aspects of Basal Cell Carcinoma
Basal cell carcinomas are common in cats and uncommon in dogs. Basal cell carcinomas in cats sometimes coexist with actinic keratosis and/or squamous cell carcinoma but most occur in regions of the skin that are not exposed to ultraviolet radiation. It is common for the overlying epidermis to be alopecic, ulcerated, or blue/black in color due to pigment within the tumor [13]. The metastatic rate of basal cell carcinomas in dogs and cats is very low.
Gross Pathology and Histopathology of Basal Cell Carcinoma
Basal cell carcinomas can be divided into three histologic types: solid basal cell carcinoma, keratinizing basal cell carcinoma (also called basosquamous carcinoma or basal cell carcinoma with follicular differentiation), and clear cell basal cell carcinoma [59].
Solid basal cell carcinomas are circumscribed, irregular masses of epithelial aggregates. Necrosis is often present in the centers of epithelial islands. The epithelial cells of basal cell carcinomas may produce mucin. Melanization of the epithelial cells is common in solid basal cell carcinomas [59].
Keratinizing basal cell carcinoma is an irregular dermal mass that is commonly ulcerated. These tumors usually do not have melanization or ulceration as described for solid basal cell carcinomas. The centers of the epithelial islands in these tumors exhibit squamous differentiation and keratinization [59].
Clear cell basal cell carcinoma is a rare variant of basal cell carcinoma seen occasionally in cats. These tumors have similar architecture to solid basal cell carcinomas, but the epithelial cells are large and polygonal with clear or finely granular cytoplasm. This appearance occurs due to the presence of numerous phagolysosomes within the cells [59].
Actinic Skin Cancers in Animals and Humans
A causal relationship between exposure to ultraviolet radiation and the development of cutaneous squamous cell carcinoma, basal cell carcinoma, and malignant melanoma is well established in humans [62]. The skin of companion animals is partially protected by their coat, but chronic solar damage can still occur in regions with little or no pigment (Fig. 10.5). Susceptible regions include the skin of the planum nasale, the abdomen, and areas of the ears, face, and limbs.
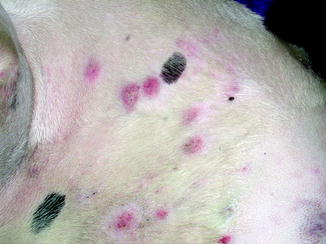

Fig. 10.5
Multifocal cutaneous squamous cell carcinoma lesions along the light skinned, thin coated trunk of a dog (Image provided by Michael Kent, DVM)
An association between sun exposure and oculofacial squamous cell carcinoma in white-faced cattle, horses, and cats has been observed [59]. There is also evidence for a similar association of sun exposure and squamous cell carcinoma in certain breeds of dogs. Dogs also have an association with solar exposure and cutaneous hemangiosarcoma. The increased frequency of this particular actinic tumor may be due to the thin epidermis of dogs allowing more dermal damage from ultraviolet radiation. In contrast to humans, basal cell carcinomas do not appear to have an increased incidence in dogs or cats in sun-exposed locations. There is also no evidence for an association between solar dermatosis and cutaneous melanoma in dogs and cats [59].
Comparative Molecular Pathology of Skin Cancer
In the modern era of oncology, it has become increasingly important to understand the molecular biology underlying the pathogenesis of each tumor. As it relates to comparative oncology, this suggests that it is not sufficient to assume that just because tumors in animals and humans have similar histopathologic features, they necessarily share the same molecular drivers of oncogenesis. However, many of the genes and pathways that are aberrantly expressed or regulated in human cancers have also been found in animals. The list of known mutations and gene expression changes seen in companion animal cancers continues to grow. The following section describes some of the biologic factors that have been shown to be associated with skin tumors in dogs and cats and the possible comparative relevance.
Mutations and altered expression of the p53 tumor suppressor gene are among the most common changes seen in cancer in animals and humans. Specific skin cancers that have been reported to altered p53 expression in dogs include papilloma, squamous cell carcinoma, and melanoma [63–68]. In canine melanoma, alterations in p53 appear to occur later in the progression of melanoma and can include gene deletion, gene silencing, disruption of p53 DNA-binding domains, or altered stability or subcellular localization of the protein. Altered p53 expression has also been shown in cats and horses with squamous cell carcinoma and horses with melanoma [67, 69].
Vascular endothelial growth factor (VEGF) is an important growth factor and regulator of angiogenesis that plays a role in a wide variety of human tumors. VEGF is expressed in canine squamous cell carcinoma but not in basal cell tumors [70]. An autocrine pathway for VEGF signaling in canine squamous cell carcinoma has been proposed [71]. In canine melanoma, one study showed that 95 % of melanoma tissues expressed VEGF and that serum and plasma levels of VEGF were higher in dogs with melanoma than a control population. Furthermore, high circulating VEGF levels correlated to shorter survival time in definitively treated dogs [72].
Bcl-2 is a key regulator of programmed cell death that has an anti-apoptotic function. Bax is a proapoptotic protein of the same family. This gene family has been shown to play a role in the growth of the circumscribed, superficial cutaneous basal cell tumors in cats, in part, due to high expression of bcl-2 and low expression of bax in basal cell tumors [73]. These tumors are slowly proliferating based on low Ki-67 labeling index but are insensitive to apoptotic stimuli due to their levels of bax and bcl-2; less than 1 % of tumor cells are found to be undergoing apoptosis by TUNEL assay [73].
β-catenin is an intercellular junction protein involved in the Wnt signaling pathway present in the epidermis of normal skin with a cytoplasmic and membrane distribution. Deregulation of the Wnt/β-catenin signaling pathway is associated with abnormal cellular proliferation and differentiation. Membrane β-catenin expression has been shown to be reduced or absent in many cases of canine squamous cell carcinoma [74]. A similar distribution is seen in human squamous cell carcinoma. A similar loss of membrane β-catenin expression has been demonstrated in canine melanoma [75].
Cyclooxygenase (Cox) is an enzyme involved in arachidonic acid metabolism leading to the formation of prostaglandins. One isoform (Cox-1) is constitutively expressed in most tissues and appears to play a homeostatic role. A second isoform (Cox-2) is an induced enzyme expressed in inflammatory and other pathologic conditions including many cancers. Overexpression of Cox-2 leads to production of high levels of prostaglandins, specifically prostaglandin E2 (PGE2). PGE2 increases angiogenesis, tumor cell proliferation, and resistance to apoptosis. Moderate to high expression of Cox-2 has been demonstrated in canine, feline, and equine squamous cell carcinoma and canine melanoma [76–79]. Cox-2 expression has also been shown to be induced in basal and suprabasal keratinocytes of dogs and cats with actinic keratosis [80].
Survivin is a protein belonging to the inhibitor of apoptosis (IAP) family and also plays a role in cell-division pathways. In normal human and canine epidermis, survivin expression is low or absent and confined to basal keratinocytes. In canine and human squamous cell carcinoma, nuclear survivin expression is present and a subset of cases show cytoplasmic survivin expression as well. Survivin is also expressed in advanced melanomas in humans and is a potential therapeutic target. A study in dogs evaluating siRNA knockdown of canine melanoma cells inhibited cell growth and increased apoptotic cell death [81].
A mutation in the Birt-Hogg-Dubé (BHD) tumor suppressor gene is associated with hereditary multifocal renal cystadenocarcinoma and nodular dermatofibrosis in German Shepherd dogs [82]. This condition is characterized by multifocal cutaneous nodules consisting of dense collagen fibers with concurrent uterine leiomyomas and multifocal tumors in the kidneys. Birt-Hogg-Dubé syndrome in humans is a rare disorder that is frequently associated with mutation to the same gene and is characterized by multiple benign skin tumors and lung tumors. Transforming growth factor-beta-1 has also been shown to be overexpressed in the cutaneous lesions of dogs with nodular dermatofibrosis [83].
Activation of telomerase is a mechanism that allows many types of neoplastic cells to avoid senescence. Telomerase levels are undetectable or low in canine somatic tissues but are elevated in 95 % of canine tumors including cutaneous tumors [84, 85].
SART-1 is a squamous cell carcinoma antigen that is recognized by cytotoxic T lymphocytes and is a potential target for immunotherapy. mRNA of the canine orthologue of SART-1 has been identified in canine squamous cell carcinoma and has a very similar sequence to human SART-1. It is not currently known whether canine SART-1 induces a T-cell response [86].
Metallothionein is an important transcription factor regulator that has increased expression in human melanoma. Metallothionein [87–89] expression has also been demonstrated in a subset of canine and feline cutaneous melanomas [90]. Cyclin-dependent kinase inhibitor 2A (also known as ink-4a) is a tumor suppressor protein. Mutations of the gene encoding ink-4a (P16Ink4A) are among the most frequent genetic abnormalities seen in human tumors, including over 50 % of malignant melanomas [91]. Loss or dramatic reduction of this protein has been demonstrated in canine melanoma [65]. The WAF1 p21 tumor suppressor gene is important for melanocyte growth and differentiation and has been seen to be mutated or poorly expressed in human melanoma. Similarly, loss, dramatically reduced expression, and altered subcellular localization of this protein have been shown in canine melanoma [65]. PTEN is a tumor suppressor gene that is mutated or silenced in many human cancers including malignant melanoma. Loss or dramatic reduction of PTEN has been demonstrated in canine melanoma [65]. N-ras is a protooncogene that when mutated is a potent inducer of tumorigenesis due to activation of growth-related signal transduction pathways. N-ras mutations have been detected in many human tumors including melanoma. N-ras mutations have also been found in canine malignant melanoma [92].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree