- Patients with type 1 diabetes usually present with classic symptoms and occasionally diabetic ketoacidosis.
- Patients with type 2 diabetes mellitus (T2DM) may be asymptomatic or present with classic symptoms.
- With advancing age, the renal threshold for glucose increases and thirst perception diminishes.
- T2DM may present with complications of diabetes which may be either microvascular or macrovascular.
- Initial diagnosis of T2DM during acute myocardial infarction or stroke is common.
- Presentation may be asymptomatic and discovered on routine examination or laboratory test.
Introduction
Diabetes has long since taken over from syphilis as the great imitator, and nowhere is this more apparent than in the wide variation of possible modes of initial presentation. The classic triad of thirst, polydipsia and polyuria accounts for only a modest proportion of new diagnoses of diabetes. The relatively acute onset of such symptoms associated with loss of weight is the hallmark of type 1 diabetes mellitus (T1DM). Ketoacidosis and hyperosmolar hyperglycemic syndrome may precipitate a dramatic presentation to emergency services. Non-specific symptoms including tiredness, general malaise and repeated or persistent skin infections may lead to a biochemical diagnosis of diabetes. Screening of at-risk groups or individuals allows early diagnosis. Regrettably, the nature of the condition is such as to allow it to remain asymptomatic for years, allowing the clinical presentation to be a long-term complication of diabetes. This could be in the form of macrovascular disease (myocardial infarction, stroke, black toe) or in the form of microvascular disease (loss of visual acuity, neuropathy). Pregnancy may cause gestational diabetes (GDM), which although may remit after delivery, does indicate a high risk for future type 2 diabetes mellitus (T2DM).
Clinical considerations at presentation
At the heart of any consultation involving the presentation of diabetes there is a patient. Depending upon prior knowledge, “diabetes” may be associated in their mind with blindness and amputation, disability and premature death. Alternatively, it may be associated with vague concepts of malaise along with lumbago or fibrositis. The patient’s beliefs and thoughts on diabetes need to be established if the diagnostic consultation is to be a therapeutic consultation. In an era of medicine by numbers, often traduced as “evidence-based medicine,” it is easy to overlook the impact of the consultation itself upon the person who will live with diabetes. “Where were you when JFK was shot?” “What was it like when you were told you have diabetes?” The moment is likely to be memorable and influential.
The therapeutic consultation will involve listening, a process that need not be unduly time consuming. “Do you know of anyone with diabetes?” “What do you know of diabetes just now?” The information received will allow the patient’s likely type of diabetes and immediate prognosis to be put into perspective. Together with other aspects of sound clinical history-taking, it will also transform that person’s view of the consultation. Patients list “listening” as the most valued attribute of a doctor. Although others may listen too, this cannot be delegated to the health care team.
At what stage of diabetes is the person in front of you? The implications for the individual who was identified on routine screening are quite different from those for the person presenting with a black toe. The former is likely to be at an early stage of a long process with good chance of modifying disease progression, whereas the latter is likely to have other tissue complications already established. Clearly, genetic susceptibility to develop complications plays a part as well as natural history time course. The former patient may never develop more than microaneurysms in the eye and be resistant to diabetic nephropathy. Even if they are to be susceptible to complications, these are amenable to intervention over a period of many years. The latter patient, however, requires clear explanation of what can be done and how future trouble can be avoided. Hippocrates summed it up nicely: “Cure sometimes, relieve often, comfort always.”
The possibility of cure should not be overlooked. Diabetes has long been regarded as incurable. However, this is not always true. At the beginning of the 21st century and with further advances in our understanding, the number of circumstances where diabetes can be cured will increase. Look out for the slatey grey person with large liver and hemoglobin level of 19 gm/dL. Hemochromatosis is rare as a cause of diabetes but it is treatable and therefore important (see Chapter 18). The person taking a combination of thiazide diuretic and beta-blocker will be pleased to have hyperglycemia at least ameliorated by use of alternative agents (see Chapter 16). Cushing syndrome may include curable diabetes (see Chapter 17). Few people on systemic steroid therapy can be taken off treatment just because of development of diabetes, but knowledge is cheering that the diabetes will go away or become much more easily controllable when the steroid course finishes. Cure of T2DM by substantial and sustained weight loss coupled with increased daily physical activity is possible for those who have the determination and willpower to change long-standing behavior patterns. Bariatric surgery produces dramatic and long-term cure of T2DM in the early years of the condition [1].
Types of diabetes
The classification of diabetes will remain the cause of much debate until the exact etiology of each subtype has been established. Currently, the paradigm is to group together those people who appear to have primary β-cell destruction as T1DM, and those who are not slim and who can be controlled at least in the early years with diet and oral agents as T2DM. The monogenic causes of diabetes are capable of precise genetic description and are clearly separate (see Chapter 15). Similarly, pancreatic disease such as chronic pancreatitis, pancreatic carcinoma and hemochromatosis is capable of precise diagnosis (see Chapter 18). T2DM, however, is a term used to describe all those conditions that do not fit into the other, more easily described categories. It is clear that more subtypes will be identified in due course.
The important practical question at the initial presentation of a person with diabetes is whether insulin therapy is necessary. In some circumstances there is no doubt, such as diabetic ketoacidosis or severe weight loss with ketonuria and glycosuria in a child (Figure 19.1). More usually in adult practice the question must be asked. Table 19.1 lays out the common and distinguishing features from the clinical history, examination and urinalysis to help the clinician come to the answer. The subsequent sections consider the separate features in context.
Figure 19.1 A 3-year-old boy before and after 3 months of insulin therapy (1922). The severe wasting of muscle and adipose tissue due to the insulin deficiency of type 1 diabetes is painfully evident in the left-hand panel. There is no more dramatic reminder of weight loss as a prominent presenting feature of type 1 diabetes especially if presentation is delayed. The speed of restoration of body mass on replacing insulin (right-hand panel) is impressive. Reproduced with permission from Eli Lilly & Co.
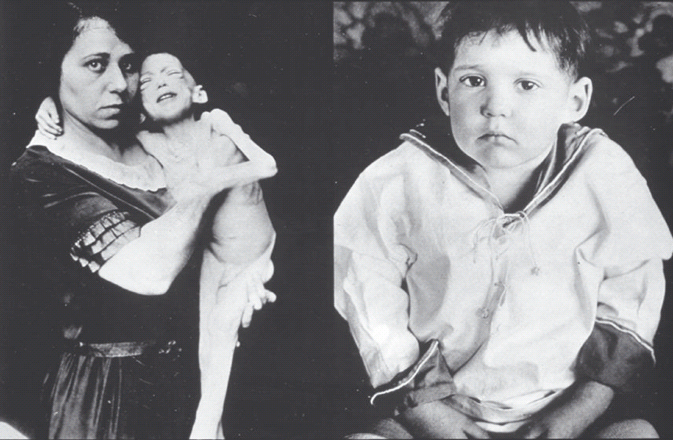
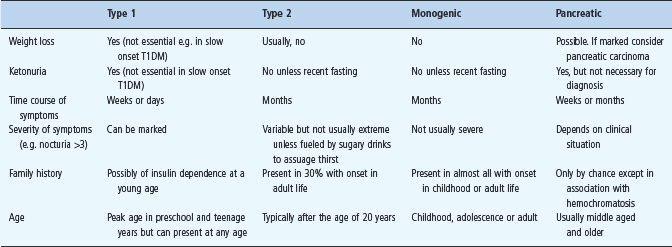
Table 19.1 Clinical features at presentation of type 1, type 2 and monogenic diabetes. This is a diagnostic guide with exceptions because of specific circumstances. It is not exhaustive and does not include rarer forms of diabetes, including syndromic diabetes.
Thirst, polydipsia and polyuria
These symptoms result from an osmotic diuresis as a consequence of hyperglycemia. The symptoms are common to all types of diabetes although the time course is likely to be shorter and the symptoms more severe in T1DM. Not infrequently, sugar-containing carbonated drinks are selected to slake thirst with resulting worsening of symptoms. A careful history documenting the time course of symptoms and any change in intake of specific drinks is important. Remembering how many times per day urine is passed is not easy, but nocturia is more clear-cut and the number of times urine is passed at night should be quantified. “Do you need to drink water when you get up at night?” is a reasonably objective measure of thirst.
For glucose to escape into the urine, plasma glucose concentration must exceed the renal threshold for tubular reabsorption of glucose and the absolute amount of glucose delivered to the renal tubules must exceed the maximum absorptive capacity. The renal threshold averages 11 mmol/L but displays a wide individual variation of around 6–14 mmol/L [2]. Additionally, the maximum absorptive capacity varies with age such that older people exhibit glycosuria at higher plasma glucose levels [3].
The rise in maximum renal tubular absorptive capacity with increasing age is clinically significant as older people will only develop osmotic symptoms at higher plasma glucose levels. Conversely, a negative urine test is even less likely to exclude a diagnosis of diabetes than in younger people. In addition to the need for higher plasma glucose levels in older people to produce osmotic symptoms, the threshold for triggering the sensation of thirst rises with advancing years [4]. This is important because, once the maximum renal absorptive capacity has been exceeded, dehydration will become considerably more advanced before thirst is sensed. These age-related changes are highly relevant to development of severe hyperosmolar states.
The presence of chronic hyperglycemia itself changes the renal sensitivity to vasopressin such that thirst is not appreciated despite rising plasma osmolarity [5]. Hence, the combination of undiagnosed diabetes and advanced age is particularly potent in delaying appropriate action to increase oral fluid intake as dehydration progresses. The clinical features identified from the history at presentation will vary in relation to the above factors. In older people, thirst may be experienced despite an osmotic diuresis and polydipsia will be absent. The most reliably quantitated feature of an osmotic presentation is therefore frequency of nocturia, and specifically an increase from habitual levels.
In children, enuresis may be the first symptom of polyuria. Sudden onset of enuresis should always prompt testing of urine for glucose. It must be noted that a urine test is entirely appropriate as an initial screen in this situation, as the absence of glucose from the urine absolutely excludes hyperglycemia as a potential cause of polyuria.
Weight loss
Establishing whether significant weight loss has occurred is the most important aspect of history-taking in those with newly presenting diabetes. Unless secondary to concurrent disease, the Clinical features at presentation of type 1, type 2 and monogenic diabetes. This is a diagnostic guide with exceptions because of specific circumstances. It is not exhaustive and does not include rarer forms of diabetes, including syndromic diabetes. symptom strongly suggests insulin deficiency and hence newly presenting T1DM. Its absence does not exclude T1DM as the speed of onset of insulin deficiency and the presence of intercurrent illness, which may have exacerbated osmotic symptoms, may mean that weight loss has not yet commenced.
Weight loss at presentation of T2DM may occur as a result of dietary restriction often undertaken because of suspicion of impending health problems. Such deliberate changes in eating habit are readily established from the history. Typically, weight does not change, or even continues to rise, prior to the symptomatic onset of T2DM.
The weight loss reflects mainly the relative loss of the anabolic actions of insulin. Muscle wasting may be prominent, especially in young men. Associated loss of muscle strength may be reported. As an anabolic hormone, insulin acts principally to inhibit protein degradation [6]. Its relative absence allows the balance between continuous protein synthesis and breakdown to be disturbed. There is an additional effect of insulin deficiency in the failure of normal promotion of lipogenesis and inhibition of lipolysis. Excess non-esterified fatty acids accumulate in plasma, forming substrate for ketogenesis. If the clinical presentation of diabetes is acute, a component of the weight loss will reflect the loss of both intracellular and extracellular water.
Blurred vision
Major changes in plasma glucose will be followed over a period of days and weeks by blurring of vision. The symptom is typically present after a relatively acute change, usually in the context of presentation of T1DM or in the specific circumstance of a hyperosmolar presentation of T2DM. It is most important to explain to the patient that the visual blurring will become worse following the relatively rapid correction of gross hyperglycemia. This explanation is vital to avoid the supposition that diabetic blindness is already progressing with consequent unnecessary worry. It is also important to prevent the unnecessary purchase of spectacles that will be redundant after the hyperglycemia is treated.
It is reasonably assumed that shifts in osmotic pressure between plasma and inside the eyeball accounts for the visual change. Certainly, this provides a practical and immediately understandable explanation. Detailed tests, however, have not to date tied down any identifiable refractive change [7].
Infections
Exposure of leukocytes to glucose concentrations above 11 mmol/L produces paralysis of phagocytic and other functions [8]. This effect, together with other possible effects upon immune function, explains the impaired ability to fight off bacterial and fungal infections. Susceptibility to viral infections appears to be little changed although clear data are lacking.
Recurrent or refractory yeast infections may draw attention to previously undiagnosed diabetes. Most frequently this involves vaginal candidiasis in women or balanitis in men. Initial control of blood glucose levels will permit clearance of the infection with continued antifungal application. Staphylococcal pustules, boils and carbuncles may be present at the diagnosis of diabetes, especially T1DM. This clinical observation was supported by a prospective study of 482 patients with skin or mucous membrane sepsis presenting to an accident and emergency department who were found to have over a threefold increased incidence of capillary blood glucose >7.8 mmol/L compared with a background population [9].
Very rare but serious infective presentations of diabetes must be considered. Necrotizing fasciitis is considerably more common in people with diagnosed and undiagnosed diabetes [10]. Fournier gangrene (gangrene of the perineum and genitalia) is associated with diabetes in almost 50% of cases [11]. The rare and often fatal facial and/or maxillary sinus fungal infection mucormycosis is most often associated with diabetes [12].
Diabetic ketoacidosis
Diabetic ketoacidosis (DKA) occurs as a result of marked insulin deficiency associated with an increase in circulating levels of counter-regulatory hormones. It is characterized by hyperglycemia, acidosis and ketonuria. It mainly occurs in patients with T1DM, but it is not uncommon in some patients with T2DM. There is a wide geographic variation in the reported incidence of DKA. For example, EURODIAB, a cross-sectional survey of 3250 people with T1DM in 29 centers in Europe, reported that 8.6% of patients had been admitted with a diagnosis of DKA in the previous 12 months [13]. In 25% of cases, DKA is the presenting feature of T1DM [14]. The overall mortality rate from DKA ranges 2–5%, but is higher in the elderly.
DKA typically presents with the symptoms of hyperglycemia (i.e. thirst, polyuria and polydipsia). Patients may also complain of malaise or lethargy and muscle cramps. Abdominal pain and vomiting may be sufficiently severe as to mimic an acute surgical problem. It is critically important to recognize this, as the administration of an anesthetic is almost invariable fatal. All doctors dealing with emergencies should be aware of the potential pitfall of missing this telltale sign of DKA.
Clinical signs include dehydration, deep, sighing respirations (air hunger or Kussmaul respiration) and a sweet-smelling fetor (like nail varnish remover) caused by the ketones on the breath. As the ability to detect the smell of ketones is genetically determined, and approximately one-third of people are unable to do this, it is important that individual doctors are aware if they are not equipped with this additional diagnostic tool. Consciousness may be clouded. If the condition has progressed to the stage of coma, the associated signs of dehydration must lead to urgent checking of blood glucose, urinary ketones and arterial blood pH in order to expedite definitive treatment.
The marked deficiency or absence of insulin in this condition means that insulin-mediated glucose uptake into tissues such as muscle, fat and liver cannot occur. In the meantime, the dysregulated secretion of counter-regulatory hormones (glucagon, growth hormone and catecholamines) enhances the breakdown of triglyceride into free fatty acids and increases the rate of gluconeogenesis, which is the main cause for the high blood glucose level in diabetic ketoacidosis. Beta-oxidation of these free fatty acids leads to formation of ketone bodies (β-hydroxybutyrate, acetoacetate and acetone). Acetone is volatile and is released from the lungs, giving the characteristic sweet smell to the breath. Metabolic acidosis ensues when the ketone bodies are released into circulation and deplete the acid buffers.
The hyperglycemia-induced osmotic diuresis further depletes sodium, potassium, phosphates and water. Patients are often profoundly dehydrated and have a significantly depleted total body potassium at presentation. Sometimes, a normal or even elevated serum potassium level is seen as a result of the extracellular shift of potassium with severe acidosis. Great care must be taken to monitor serum potassium levels repeatedly once insulin treatment is started as the concentration can drop precipitously.
Hyperosmolar hyperglycemic syndrome
Hyperosmolar hyperglycemic syndrome (HSS) occurs exclusively in patients with T2DM. Often there is a history of several days of ill health. The principal clinical feature is profound dehydration. Confusion is usual, and focal neurologic symptoms such as weakness on one side or hemi-sensory abnormalities may develop and be easily confused with stroke. HSS was previously termed hyperosmolar non-ketotic coma. This terminology has been changed as coma is a relatively rare feature (<10%) and mild ketosis may be present at diagnosis.
HSS shares many features in common with DKA, the major exceptions being the absence of significant ketoacidosis. This is likely because of the residual low level insulin secretion, which suppresses lipolysis sufficiently to avert ketogenesis but not sufficient to prevent hyperglycemia. Additionally, hyperosmolarity itself may decrease lipolysis, limiting the amount of free fatty acids available for ketogenesis. HSS accounts for 10–30% of hyperglycemic emergencies. As the prevalence of T2DM rises inexorably, it is becoming an increasingly common hospital admission. Up to two-thirds of those affected have not previously been diagnosed as having diabetes.
Macrovascular presentations
Acute myocardial infarction
As the risk of ischemic heart disease is linearly related to fasting and post-prandial blood glucose concentrations, it is not surprising that both impaired glucose tolerance (IGT) and diabetes are over-represented in populations presenting with acute myocardial infarction (AMI) [15]. Consequently, T2DM frequently presents for the first time at hospitalization for AMI.
This presentation is complicated by stress hyperglycemia resulting from the catecholamine and cortisol elevations. Although this may cause problems for the purist wishing to evaluate an effect of diabetes per se, from the perspective of the patient with a life-threatening condition exacerbated by dysglycemia, exact definitions of diabetes are not relevant. Stress hyperglycemia and established diabetes have similarly increased mortality from AMI. In a New York municipal hospital cohort of patients with AMI, 3-year mortality was 52% in those with stress hyperglycemia (defined as admission blood glucose >7.0 mmol/L) compared with 42% in those with diabetes [16]. The 3-year death rate in those with normal glucose levels was 24% in the same study. A meta-analysis has confirmed this effect, with a 3.9-fold increase risk of death associated with stress hyperglycemia compared with a 1.7-fold increased risk of death associated with established diabetes [17]. In this context, one of the most important findings of the DIGAMI study is often overlooked. The effect of reasonable glycemic management (blood glucose <10 mmol/L) for those with no prior insulin therapy and stratified as having low coronary risk factors produced a 52% improvement in mortality [18]. This group would have included those with stress hyperglycemia. In contrast, the DIGAMI study showed no significant benefit of acute blood glucose control for individuals previously treated with insulin.
Estimates of the incidence of stress hyperglycemia at presentation of AMI range 10–16% [19,20]. This compares with estimates of prevalence of diabetes at presentation of AMI of 25–32% [19,21,22]. Variation in these figures is likely to reflect the background prevalence of IGT and T2DM in the population, as well as increased awareness and effective screening processes to identify previously undiagnosed T2DM.
Good clinical practice demands measurement of plasma glucose on diagnosis of an acute coronary syndrome. If plasma glucose is raised (7 mmol/L may be quoted, but in the individual case interpretation depends upon time since last meal), then both fasting plasma glucose and HbA1c should be measured. Raised plasma glucose should indicate a need for particular attention to adequate glucose control during the acute event. Given that the HbA1c result is unlikely to be available immediately, hyperglycemia indicates a need for rapid control in the acute situation when the first few hours are critical. A fasting plasma glucose of >5.6 mmol/L during the acute admission and/or admission plasma glucose of >7.8 mmol/L yielded a sensitivity of almost 90% and a positive predictive value of 44% for detecting diabetes [23].
Where there is diagnostic uncertainty, targeted screening in the post-acute setting with a standard 75 g oral glucose tolerance test (OGTT) is acceptable. But when is the optimal time to perform this test? In a group of AMI patients with no previous diagnosis of diabetes, both pre-discharge and 6 weeks post-discharge OGTTs were performed and correlation with pre-discharge OGTT was good [24]. There was 49% concordance between classifications to which each patient was assigned in both OGTTs. The best predictor of abnormal glucose handling (IGT or diabetes) being diagnosed at 3 months was observed to be the 60-minute blood glucose level during the pre-discharge OGTT.
Acute stroke
The prevalence of previously diagnosed diabetes in patients with acute stroke is 8–28% but an additional 6–42% have unrecognized pre-existing dysglycemia [25]. Plasma glucose at presentation is a major prognostic factor. One series of 86 patients with acute stroke demonstrated that full functional recovery at 4 weeks was restricted to those with presenting blood glucose levels <8 mmol/L [26]. None of the individuals with a raised presenting plasma glucose regained full function by 4 weeks. The extent to which this reflects the metabolic stress response in proportion to the severity of the cerebrovascular insult as opposed to hyperglycemia itself impairing subsequent recovery from ischemic damage cannot be ascertained from these observational data.
The observations on poorer outcome in those who had stress hyperglycemia following AMI have been reproduced in respect of acute stroke disease. In a systematic review of observational studies examining the prognostic significance of hyperglycemia in acute stroke, the unadjusted relative risk of in-hospital or 30-day mortality was 3.07 (95% CI 2.50–3.79) in non-diabetic patients with admission plasma glucose level >6–8 mmol/L and 1.30 (95% CI 0.49–3.43) in those with known diabetes [27]. The relative risk of poor functional outcome in hyperglycemic non-diabetic patients was 1.41 (95% CI 1.16–1.73). It appears that sudden increase in plasma glucose levels impair tissue function more in those individuals who have not been habituated to hyperglycemia.
Persistent hyperglycemia (defined as blood glucose >7.0 mmol/L) in the 72 hours after acute stroke was found to be associated with an increase in infarct size, measured using magnetic resonance imaging, and worse stroke outcome [28]. Nonetheless, there are currently no satisfactory outcome studies of control of plasma glucose upon the outcome of stroke [29]. The largest study to date, which included 993 patients, failed to achieve control of plasma glucose at 24 hours [30]. Importantly, no assessment has yet been conducted of plasma glucose control during the first few hours after presentation with acute stroke, and it is likely that it is in this window of time that this particular presentation of hyperglycemia may most beneficially be managed.
Microvascular presentations
Eye presentations
Symptomatic loss of vision may occasionally be the presenting feature of T2DM, where hyperglycemia has been present for an uncertain number of years, silently causing tissue damage and retinopathy. Loss of vision as a diagnostic event is most often a consequence of macula edema but may also be secondary to vitreous hemorrhage. Central or branch retinal vein occlusion is more common in diabetes and may also cause symptomatic presentation of the condition.
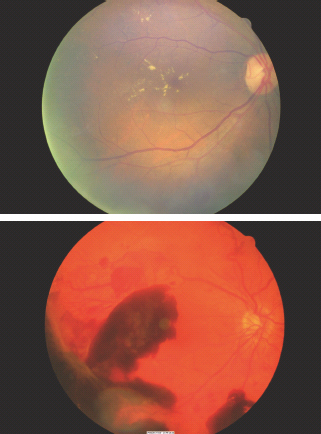
Around the time of diagnosis of T2DM, marked retinopathy with cotton wool spots or intraretinal microvascular abnormalities (IRMA) was found to be present in 8% of men and 4% of women in the UK Prospective Diabetes Study (UKPDS) [31]. The critical importance of arranging full retinal examination, preferably by digital retinal imaging, is illustrated in Figure 19.2. Approximately 1% of individuals presenting with symptomatic T2DM have sight-threatening retinopathy at that time. Very early recognition is essential as the initial treatment of the diabetes will decrease blood glucose levels, cause retinal blood flow to return acutely to normal levels and may result in marked worsening of the retinopathy.
Figure 19.2 Upper panel: immediate laser therapy was required for the macular edema associated with the severe exudative maculopathy present at the time of diagnosis. Lower panel: new vessels are present both arising from the optic disk and from the peripheral retina. Bleeding from the latter caused the prominent pre-retinal hemorrhages which obscure the fovea and in this case caused presentation because of loss of visual acuity.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree