Summary of Key Points
- •
Most people with lung cancer are symptomatic at the time of initial presentation; however, between 5% and 15% of people may be asymptomatic.
- •
Alarming symptoms for lung cancer like cough, hemoptysis, dyspnea, chest pain and weight loss should be timely recognized.
- •
No specific clinical manifestations exist to guide and help the physician distinguish between specific histologic subtypes.
- •
Increased awareness of cough, one of the most common lung cancer symptoms, may help in the earlier detection of lung cancer.
- •
Hemoptysis is the only symptom that typically causes people to be prompt in reporting to their primary care physician.
- •
Lung cancer is one of the most common etiologies for a malignant pleural effusion.
- •
Lung cancer is the cause of superior vena cava syndrome in about 50% of cases.
- •
Lung cancer is the most common cause of brain metastases.
- •
Paraneoplastic syndromes are not uncommon in lung cancer and may be the first clinical manifestation of the disease.
- •
Certain clinical as well as molecular factors may have a potential prognostic and/or predictive role for guiding lung cancer patients’ personalized care.
Most people with lung cancer are symptomatic at the time of initial presentation; however, between 5% and 15% of people will be asymptomatic at the time of diagnosis. Patients who do have symptoms at the time of diagnosis often have advanced disease and therefore have a poorer prognosis, with an overall 5-year survival rate of 15% or less. In order to improve lung cancer survival, early detection with a screening method should be considered for asymptomatic people at highest risk. Indeed, screening of asymptomatic high-risk individuals (defined as current or former smokers who are aged 55 to 74 years old, with a smoking history of a minimum of 30 pack-years) with use of low-dose computed tomography (CT) has been shown to be efficient not only for diagnosing lung cancer at an early stage but also for substantially reducing lung cancer–specific mortality.
In contrast, people in whom lung cancer is detected during screening trials will usually be asymptomatic because these tumors are usually very small and peripherally located. Outside of screening programs, lung cancer in most asymptomatic people will be diagnosed coincidentally (e.g., on a chest x-ray for other indications or for a preoperative examination). It is of utmost importance for every clinician to be aware of all possible lung cancer symptoms at the time of initial presentation, and not just the so-called alarming symptoms. These alarming symptoms—cough, hemoptysis, dyspnea, chest pain, and weight loss—are mostly a result of local intrathoracic tumor growth, but may be further triggered by local–regional intrathoracic invasive growth and also by the development of extrathoracic metastases ( Table 20.1 ). Timely recognition of alarming symptoms can be difficult because they often develop in older patients (aged 60 years or older) who are current or former smokers and who may also have comorbidities such as chronic obstructive pulmonary disease (COPD) or cardiac disease (e.g., heart failure, angina pectoris). In certain cases of lung cancer, symptoms at presentation may be linked to specific paraneoplastic manifestations that, if unrecognized, may cause diagnostic dilemmas and further delay the diagnosis of lung cancer. The more common symptoms that occur at initial presentation of a person with lung cancer will be further described later in the chapter. Unfortunately, there are no specific clinical manifestations to guide and help the physician distinguish between specific histologic subtypes of lung cancer. Physicians should also consider that as a result of a lung cancer diagnosis, other symptoms may arise that are not addressed in this chapter, including those caused by iatrogenic complications of surgery, systemic treatment or radiotherapy, or other symptoms that may only occur specifically during the end-of-life care of patients with lung cancer. Also addressed are a variety of clinical and molecular factors with potential use for early diagnosis and management of lung cancer, as well as for prognostication.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Symptoms and Signs of Local Tumor Growth
Cough
One of the most common symptoms related to lung cancer is cough, which is reported in up to 60% to 70% of people at the time of diagnosis. Increased awareness of cough may help in the earlier detection of lung cancer, resulting in better outcomes, as was demonstrated in a recent United Kingdom Cough Awareness Campaign. Cough is most often caused by larger, centrally located bronchial mucosa invading lung tumors, but may also be present in smaller, peripheral lung tumors. A copious production of thin, colorless sputum (bronchorrhea) may be found in some patients with lung adenocarcinoma with a predominant lepidic growth pattern, but this is rare. Smaller but predominantly endobronchially located lung tumors may also cause cough. This cough may be either dry or nonproductive, but may be productive if respiratory infections occur as a consequence of the obstruction. Because cough is also a predominant symptom in other lung diseases such as COPD, it is sometimes difficult to recognize cough as a presenting symptom of lung cancer. When recording the medical history of the patient, special attention should be given to the changing cough pattern that may occur in patients with COPD or active smokers in whom lung cancer develops. The failure of an acute COPD exacerbation to resolve with adequate therapy should raise suspicion of an underlying respiratory malignancy.
Hemoptysis
Hemoptysis is not only a common symptom in up to one-third of people at the time of lung cancer diagnosis, but is also the only symptom that typically causes people to be prompt in reporting to their primary care physician. Approximately 20% of all cases of hemoptysis are associated with lung cancer; therefore, the occurrence of hemoptysis in a patient presenting to a primary care physician should always lead to further investigation, starting with chest x-rays. Hemoptysis usually results from tumor necrosis, growth of new blood vessels in and around the tumor (neovascularization), and bronchial mucosa ulceration with erosion and invasion of bronchopulmonary vessels. It may also be caused by an obstructive pneumonia or by paraneoplastic pulmonary embolism. At presentation, hemoptysis may vary from mild (blood-streaked sputum) to moderate and severe blood loss. Fortunately, severe or massive hemoptysis (more than 200 mL of blood expectorated at once or over the course of 24 hours or 5–10 mL/h of blood expectorated over 24 hours) occurs rarely at initial presentation, but it may become an increasingly life-threatening problem during the palliative treatment phase of an advanced lung cancer. Treatment of massive hemoptysis at the time of diagnosis of lung cancer of an unknown stage or of a potentially curable, newly diagnosed lung cancer will require prompt securing of the airways by endotracheal intubation and maintaining of optimal oxygenation before more definitive alleviation of the hemoptysis by either endobronchial therapy or by urgent surgical intervention can be offered. Moderate hemoptysis caused by tumors that cannot be reached by bronchoscope can be treated with bronchial artery embolization. For all other cases of endobronchial tumors causing hemoptysis, several endobronchial therapeutic modalities exist, ranging from photocoagulation with neodymium:yttrium aluminum garnet (Nd:YAG) laser to electrocautery to argon plasma coagulation. For distal or parenchymal-situated unresectable lung tumors, external-beam radiotherapy may be recommended. Endobronchial brachytherapy has been used for the palliative treatment of hemoptysis caused by endobronchially visible tumors, and the combination of high-dose-rate brachytherapy with external-beam radiotherapy demonstrated better symptom control than with external-beam radiotherapy alone. In a recent Cochrane meta-analysis, external-beam radiotherapy alone was found to be more efficient for palliation compared with endobronchial brachytherapy alone, although there was insufficient evidence to support the superiority of the two modalities combined for palliative symptom relief compared with external-beam radiotherapy alone.
Chest Pain
People with early-stage lung cancer may note vague, persistent chest pain or chest discomfort, even though no invasion of the chest wall, mediastinum, or pleura can be found. The true origin of this pain sensation is not completely understood, as no pain fibers are present in the lung parenchyma. Peribronchial autonomic nerves are able to transmit sensations of discomfort via the vagus nerve, which may also cause rare craniofacial pain sensations in nonmetastatic lung cancers. When further tumor growth occurs with local-regional invasion, such as in the pleura, mediastinum, or chest wall, more severe local pain symptoms may occur, requiring adequate analgesic treatment in combination with tumor-directed therapy.
Dyspnea, Stridor, Wheezing
Dyspnea is a common presenting symptom of lung cancer, occurring in up to 60% of people. The causes of dyspnea are often multifactorial, related to the increasing tumor volume, endobronchial tumor obstruction causing parenchymal atelectasis, lymphangitic tumor spread in a lobe or the entire lung, or pulmonary artery embolism. When lung cancer starts its local–regional invasion into the trachea, pericardium, and pleura, dyspnea may become more severe. Besides the tumor-related causes of dyspnea, there may be other potential aggravating causes, particularly in people with lung cancer who have COPD or cardiac conditions. When a tumor occludes the lower trachea or a major central airway, an acute feeling of breathlessness can occur along with the typical sound of stridor (in cases of severe occlusion of the airway or trachea) or unilateral monophonic wheeze (in cases of left- or right-sided main airway subocclusion). Standard treatment of the underlying cancer in people with early-stage or locally or regionally advanced disease will treat the dyspnea as well. For people with more advanced and symptomatic lung cancer, early palliative treatment of dyspnea (home oxygen therapy for hypoxemia, opioids, or inhaled furosemide) should be considered.
Symptoms and Signs of Invasive Local–Regional or Intrathoracic Spread
Hoarseness
The left vocal cord is stimulated by the left recurrent laryngeal nerve, which passes deep into the left thoracic cavity and under the aortic arch before again climbing up to the left vocal cord. Enlarged lymph nodes in the aortic pulmonary window or a large, invasive tumor to the left of the aortic branch may cause left recurrent nerve entrapment, resulting in nerve palsy and vocal cord paralysis. This vocal cord paralysis—occurring in fewer than 10% of people with lung cancer—results in hoarseness and sometimes also cough and aspiration. On a combination 18 F-2-deoxy- d -glucose (FDG)-positron emission tomography (PET)-CT, the internal laryngeal muscles on the opposite side of the entrapped recurrent laryngeal nerve (usually the left one) may present with false-positive increased FDG uptake because of the compensatory laryngeal muscle activation caused by the contralateral paralyzed vocal cord. Entrapment of the right recurrent laryngeal nerve by lung tumor tissue occurs much less frequently because this nerve does not extensively run through the right side of the chest.
Pleural Effusion
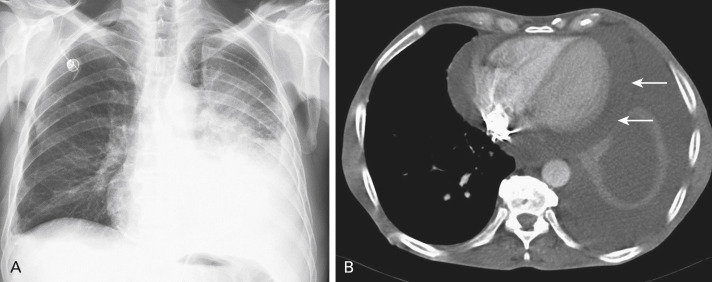
Lung cancer is one of the most common etiologies for a malignant pleural effusion. Malignant pleural fluid accumulation will eventually develop in 7% to 23% of people with lung cancer, but not all of them will be symptomatic at the time of diagnosis. Patients with a pleural fluid accumulation will generally report dyspnea, cough, chest pain, fatigue, and weight loss. The accumulation of malignant pleural fluid may be by direct invasion of the tumor into the pleura or by metastasis into the pleura ( Fig. 20.1 ). Pleural fluid accumulation may also have other causes in people with lung cancer, and these causes should be excluded: chylothorax by lymphatic obstruction or nonmalignant causes such as heart failure, pleuropulmonary infection, pulmonary infarction, and cirrhosis. Diagnostic thoracocentesis is therefore necessary to document the presence of malignant cells. In 40% to 50% of cases, the results of cytology examination will be false-negative and diagnostic medical thoracoscopy should be done to obtain a new sampling of pleural fluid combined with pleural biopsy to be examined. When the pleural effusion is confirmed as malignant, the lung cancer should be classified as stage IV (M1a), which is associated with a poor prognosis. Besides systemic therapy (chemotherapy, targeted therapy), treatment of malignant pleural effusion consists of fluid drainage and pleurodesis. Optimal treatment depends on an individual patient’s symptoms, performance status, and prognosis. For patients with a very poor prognosis (less than 3 months), repeated thoracenteses may be performed to alleviate dyspnea and pain. However, for most patients with lung cancer, a more definitive therapy for the malignant pleural effusion should be planned, either by talc slurry instillation via chest tube, thoracoscopy, or by insertion of an indwelling pleural catheter. The latter procedure is necessary in the event of lung entrapment by widespread pleural involvement.
Pericardial Effusion
Pericardial effusion occurs in 5% to 10% of people with lung cancer ( Fig. 20.1 ). Pericardial invasion by malignant cells occurs either by direct tumor invasion or by hematogenous or lymphatic spread of cancer cells. Patients who have pericardial effusion at presentation will either be asymptomatic (with only radiographic documentation of the presence of pericardial fluid) or they will report symptoms such as increasing dyspnea (up to grade 3/4), orthopnea, anxiety, palpitations, and retrosternal pain. On physical examination, specific signs of right-sided heart failure, arrhythmias (atrial fibrillation), and pericardial tamponade (pulsus paradoxus) may be found. Cardiac tamponade should be regarded as a life-threatening condition requiring immediate intervention. A suspected diagnosis of severe pericardial effusion should prompt investigation by echocardiography to document this effusion. When a right-sided ventricular collapse is found, urgent pericardiocentesis should be performed to provide relief. Following initial puncture, a pericardial catheter may be inserted for further fluid drainage. Recurrence of fluid accumulation after pericardial drainage has been performed may occur in one-third of patients. After recurrence, a new pericardial puncture may be performed together with the instillation of a sclerosing agent (e.g., cisplatin, mitoxantrone). Another treatment approach for patients with a better prognosis and a refractory pericardial effusion may be video-assisted surgical thoracoscopy with a pericardiotomy (pericardial window).
Superior Vena Cava Syndrome
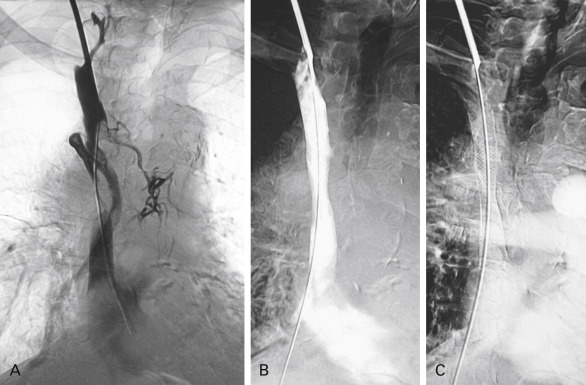
Obstruction or compression of the venous return from the head via the superior vena cava is a common complication of lung cancer. However, superior vena cava syndrome (SVCS) is rarely present (fewer than 5% of cases) at the time of initial lung cancer diagnosis. Lung cancer is the cause of SVCS in about 50% of cases, but other intrathoracic malignancies such as lymphoma, primary mediastinal tumors, and metastatic tumor in the mediastinum should be excluded as the cause. SVCS results from a growing right upper lobe lung tumor that extends centrally to the superior vena cava or by growing, malignant right paratracheal lymph nodes. An intraluminal thrombus may also form. Nonsmall cell lung cancer (NSCLC) causes SVCS more frequently than small cell lung cancer (SCLC). The clinical presentation of SVCS typically involves swelling of the head and neck, edema of the eyelids, distention of the veins in the neck and on the chest wall, cough, breast swelling, dizziness, headache, blurred vision, dyspnea, dysphagia, and chest pain. When tumor growth is aggressive, symptoms may appear more rapidly because of lack of time for collateral circulation to develop proximal to the venous obstruction, particularly when the obstruction is situated above the level of the junction with the azygos vein. Diagnosis of SVCS can be easily documented by chest CT ( Fig. 20.2 ), but a histologic diagnosis of the underlying cancer is mandatory before treatment can be initiated. Treatment of SVCS consists of alleviating symptoms as well as treating the underlying lung cancer. In the case of a local–regionally advanced lung cancer, chemoradiation therapy can be initiated. For an advanced lung cancer (especially for a SCLC), chemotherapy may begin immediately. When the patient is highly symptomatic, more rapid symptom relief can be achieved by placement of an endovascular stent than by chemoradiation therapy. Patients who present with stridor as a result of central airway obstruction or severe laryngeal edema, as well as patients who are in a coma caused by cerebral edema, should be immediately treated with endovascular stenting. This procedure can be performed with use of different types of stents (e.g., stainless steel stents such as Gianturco, Wallstent, or Palmaz); however, nitinol stents currently appear to be more suitable for the safe and efficient endovascular management of SVCS. Success rates associated with first stenting have ranged from 80% to 95%. Although the average risk of SVCS recurrence after stenting is 10% to 14%, recurrence can almost always be resolved by a new stenting procedure.
Pancoast Syndrome
A lung cancer that grows in the apex of the upper lobe toward the superior sulcus, ribs, and vertebrae will cause pain in the shoulder, scapula, and chest wall. Invasion of the brachial plexus (specifically the lower nerve roots of the ulnar nerve) will result in radiating pain and muscle wasting in the arm and hand. Horner syndrome, by invasion of the sympathetic chain and stellate ganglion, results in ptosis, miosis, and hemifacial anhidrosis, and may be part of the so-called Pancoast syndrome. Typically, patients with Pancoast syndrome will have consulted other specialists before a final diagnosis of lung cancer is made, sometimes with a delay as long as 1 year from the time pain first occurred. Most people with lung cancer who present with Pancoast syndrome have NSCLC. Pancoast syndrome as an initial clinical presentation of lung cancer occurs in about 4% of cases.
Dysphagia
Dysphagia may occur when the esophagus becomes obstructed by enlarged mediastinal lymph nodes or by a lung tumor invading the esophagus. Recurrent laryngeal nerve palsy may also cause dysphagia because of dysfunction of the laryngeal swallowing mechanism. Patients will typically note increasing difficulty swallowing and may subsequently become incapable of swallowing. Therapy consists of treatment of the underlying local–regionally invasive lung cancer and temporary parenteral feeding, if necessary. Occasionally, palliative esophageal stenting is needed.
Diaphragmatic Paralysis
When the phrenic nerve gets trapped by a growing primary tumor or by bulky lymph nodes (typically originating from the aortic–pulmonary window lymph nodes), the diaphragm may become paralyzed, resulting in an increase in dyspnea. Lung cancer invading the phrenic nerve is therefore an indication of locally advanced disease and should be staged as a cT3 tumor according to the International Association for the Study of Lung Cancer (IASLC) tumor, node, metastasis (TNM) classification eighth edition.
Symptoms and Signs of Metastatic Spread
Bone Metastases
Bone metastases develop in approximately 30% to 40% of people with advanced NSCLC, with metastases either present at the time of diagnosis or developing during the course of the neoplastic disease. Compared with bone scans, PET has similar sensitivity (at least 90%), but a higher specificity (at least 98%) and accuracy (at least 96%), and is therefore considered superior for detecting bone metastases. Therefore, if no abnormality in bones is found on a PET scan of a patient who has no signs or symptoms suggestive of bone metastases, a bone scan is not needed. Lung cancer metastases to bone are predominantly lytic.
Bone metastases cause significant pain and morbidity and are characterized by various skeletal-related events, including pathologic fractures, spinal cord compression, the need for radiation or surgery of the bone, and hypercalcemia of malignancy. Periosteal inflammation and elevation is the mechanism that most frequently causes pain from bone metastases. Pain that cannot be controlled with nonsteroidal anti-inflammatory drugs should be managed with narcotic analgesics. Most patients with symptomatic bone metastases achieve some pain relief with a low-dose, brief course of radiation therapy, as demonstrated in a trial from the Radiation Therapy Oncology Group (RTOG 97-14), which included patients with breast cancer or prostate cancer. In this trial, the efficacy of 8 Gy in a single fraction was comparable to that of the standard treatment course of 30 Gy delivered in 10 treatment fractions over 2 weeks in terms of response rates and the incidence of subsequent pathologic fractures. However, the retreatment rate was significantly higher in the 8-Gy arm (18% vs. 9%; p < 0.001). Stereotactic ablative radiotherapy (SABR) has emerged as a new treatment for bone metastases, and several randomized trials have shown promising results. In particular, the focal nature of SABR provides an otherwise unavailable noninvasive treatment option for previously radiated spinal metastases. For select patients with weight-bearing bone metastases at special risk of pathologic fracture, surgical operation may be considered. Vertebral augmentation procedures (kyphoplasty and vertebroplasty) are also important modalities when treating symptomatic vertebral compression fractures. In addition to the immediate pain relief, these procedures have several advantages, including applicability in previously radiated sites, the possibility of outpatient care, and obtaining of tissue biopsy specimens. Bisphosphonates (pamidronate and zoledronic acid) play an important role by preventing bone resorption at sites of bone remodeling. In one study, zoledronic acid was associated with a significantly lower incidence of skeletal-related events among patients with NSCLC with bone metastasis. Denosumab, another drug in a novel class of bone-targeting agents, is designed to inhibit receptor activator of nuclear factor kappa-B ligand. Compared with zoledronic acid, denosumab prolonged overall survival of patients with NSCLC with bone metastases.
Brain Metastases
Lung cancer is the most common cause of brain metastases. Metastases to the brain are usually symptomatic, and more than two-thirds of patients with brain metastases have some neurologic symptoms during the course of their illness. The clinical manifestations of brain metastases are variable depending on the location of the lesion and the degree of associated edema. Headache is a common presenting symptom and occurs more often with multiple metastases. Focal or generalized seizures have occurred in approximately 10% of patients by the time of presentation. Other symptoms of brain metastases include nausea and vomiting, focal weakness, confusion, ataxia, or visual disturbance. The signs and symptoms of brain metastases are often subtle; therefore, brain metastases should be suspected in all patients with lung cancer in whom neurologic symptoms develop. Magnetic resonance imaging (MRI) is currently the criterion standard for the diagnosis of brain metastases.
Corticosteroids can rapidly decrease the symptoms associated with brain metastases by decreasing peritumoral edema. Patients with seizures should be treated with antiepileptic medication. Subsequent treatment should be used according to size, number, and location of lesions as well as the extracranial disease status and performance status of the patient. Treatment modalities for brain metastases include whole-brain radiotherapy, stereotactic radiosurgery, and surgical resection. Stereotactic radiosurgery should be considered before whole-brain radiotherapy for patients with one to three brain metastases.
Leptomeningeal carcinomatosis is not rare in NSCLC and continues to be a devastating end-stage complication of the disease. Further improvements in systemic treatment may lead to prolonged survival for more patients with stage IV disease; therefore, when all existing therapies have failed in these patients, leptomeningeal carcinomatosis may be more likely to develop. The most common ways for malignant cells to gain access to the subarachnoid space are by direct extension from preexisting tumors or by hematogenous dissemination. A high index of suspicion is required to make an early diagnosis of leptomeningeal carcinomatosis as there is a variety of neurologic manifestations. Headache, changes in mental status, cranial nerve palsies, back or radicular pain, incontinence, lower motor neuron weakness, and sensory abnormalities are typical symptoms. The most informative diagnostic tool in the evaluation of leptomeningeal carcinomatosis is lumbar puncture. The opening pressure should be measured and cerebrospinal fluid sent for cytologic examination, cell count, and measurement of protein and glucose. Positive findings on cerebrospinal fluid cytology examination are found on initial lumbar puncture in 50% of patients with leptomeningeal carcinomatosis and in approximately 85% of patients who have three high-volume lumbar punctures. Therefore, patients with clinical symptoms and signs suggestive of leptomeningeal carcinomatosis should have repeated lumbar puncture if the first cytology evaluation yields negative results.
Leptomeningeal carcinomatosis is a particularly difficult challenge in the treatment of cancer. Intrathecal chemotherapy has been the mainstay of treatment, even though the extent of its benefit has not been proven in randomized clinical trials. A ventriculoperitoneal shunt is also an effective palliative tool to decrease intracranial pressure. Given the dismal treatment outcome and prognosis of patients with leptomeningeal carcinomatosis, new therapeutic agents or strategies are urgently needed.
Spinal Cord Metastases or Spinal Compression
Spinal cord metastases or compression can be classified anatomically as intramedullary, leptomeningeal, and extradural. Extradural compression includes several mechanisms, such as continued growth of bone metastases into epidural space, blockage of neural foramina by a paraspinal mass, and destruction of vertebral bone. At the time of presentation, 90% of patients have local or radicular pain, and up to 50% of patients may have paralysis, sensory loss, and sphincter dysfunction. If the clinical suspicion of spinal cord compression is high, immediate high-dose dexamethasone should be administered before compression is confirmed radiographically. Radiotherapy is the mainstay of treatment for spinal cord metastases and should be started immediately after MRI confirmation of spinal cord compression. For patients with lung cancer who have symptomatic epidural spinal cord compression and good performance status, a neurosurgical consultation is recommended and, if appropriate, surgery should be performed immediately, followed by radiotherapy.
Liver and Adrenal Gland Metastases
Liver involvement occurs frequently with lung cancer. Patients may have upper quadrant or epigastric discomfort as a result of large metastases. Most liver metastases are asymptomatic, and some patients experience vague symptoms such as fatigue, weight loss, and nausea. Liver dysfunction occurs only in the presence of extensive metastases.
The type of cancer most often associated with adrenal metastases is lung cancer, followed by gastric cancer. Adrenal metastases are usually detected on CT scans, and bilateral metastases appear in approximately half of patients with lung cancer. In most cases, these lesions are asymptomatic, although large metastatic masses may cause pain. Adrenal insufficiency is rare even in bilateral metastases because functional adrenal cortical loss occurs only when more than 90% of the adrenal gland has been destroyed. However, adrenal insufficiency should be suspected in patients who present with appropriate clinical symptoms and bilateral adrenal metastases. Anecdotal reports have suggested that long-term survival can occur after resection of isolated adrenal metastasis from NSCLC, but this has not been confirmed by randomized clinical trials and the chance of selection bias should be considered.
Other Metastatic Sites
Lung cancer metastases may occur at other sites, such as skin, soft tissue, pancreas, intraabdominal lymph nodes, bowel, ovaries, and thyroid. Management of these metastatic sites is primarily based on the patient’s symptoms.
Paraneoplastic Syndromes
Paraneoplastic symptoms are not uncommon in lung and other cancers, and can sometimes be the first clinical manifestation of the disease. Paraneoplastic symptoms are generally referred to as effects from the cancer that are not directly caused by invasion into vital organs, obstruction, or space-occupying effects from the primary cancer or remote metastasis. Lung cancers have long been associated with paraneoplastic effects, which encompass a large spectrum of anatomic phenomena. They include a variety of endocrine, neurologic, dermatologic, and other body function disturbances that are indirect results of the cancer and not a result of the direct presence of cancer cells. Paraneoplastic phenomena are not specific to lung cancer, although the frequency of involvement is variable among tumor types. For instance, hypertrophic pulmonary osteoarthropathy and clubbing occur more often with lung and thoracic cancers than with other primary cancers. In addition, certain lung cancer subtypes may be more related to paraneoplastic phenomena than others, in particular SCLC, carcinoid tumors, and other neuroendocrine cancers. Paraneoplastic syndromes may be classified in several ways ( Table 20.2 ) and in some cases reflect common or similar pathogenic mechanisms as well as organ of involvement.
| Classification | Paraneoplastic Phenomena |
|---|---|
| Dermatologic or musculoskeletal | Hypertrophic pulmonary osteoarthropathy Digital clubbing Dermatomyositis/polymyositis |
| Endocrine or metabolic | Syndrome of inappropriate antidiuretic hormone secretion (SIADH) Hypercalcemia Cushing syndrome Carcinoid syndrome |
| Neurologic | Lambert-Eaton myasthenic syndrome Cerebellar ataxia Sensory neuropathy Limbic encephalitis Encephalomyelitis Autonomic neuropathy Retinopathy Opsomyoclonus |
| Hematologic | Anemia Leukocytosis Thrombocytosis Eosinophilia |
| Constitutional | Anorexia Weight loss Asthenia |
Dermatologic or Musculoskeletal Disorders
Hypertrophic Pulmonary Osteoarthropathy and Digital Clubbing
Digital clubbing, which manifests early with the loss of angle between nail and nail fold, has long been acknowledged as a possible sign of lung cancer. In a review published in 2009, authors reported that digital clubbing was found in up to 10% of patients with lung cancer and in patients with tumors metastatic to the lung. Digital clubbing is associated with hypertrophic pulmonary osteoarthropathy, a condition characterized by periosteal and subperiosteal new bone formation along the shaft of long bones and the phalanges. Clinically, patients often report symmetrical, painful arthropathy of the wrists, ankles, knees, and elbows. Simple radiographic examination of the long bones may show typical periosteal new bone formation, and a bone scan usually confirms bilateral diffuse uptake by the long bones. Symptoms of hypertrophic pulmonary osteoarthropathy may respond completely to surgical resection; however, for patients who are not surgical candidates, symptomatic treatment includes specific systemic treatment of the cancer in addition to bisphosphonates; analgesics, including opiates and nonsteroidal antiinflammatory agents; and, occasionally, palliative radiation therapy.
Rare Skin Disorders
Tripe palm is a rare paraneoplastic syndrome associated with lung cancer, presenting with symptoms of thickened velvety palms and pronounced dermatoglyphics. It can occasionally occur with acanthosis nigricans, another paraneoplastic skin condition that manifests as gray-brown hyperpigmented skin plaques. Another rare paraneoplastic syndrome linked to lung cancer is erythema gyratum repens, which is usually associated with substantial disease burden and is a cutaneous eruption with a unique wood-grain pattern morphology.
Dermatomyositis
Dermatomyositis is an inflammatory myopathy associated with skin changes. A typical sign of dermatomyositis is a heliotrope rash (blue-purple discoloration named after the heliotrope plant) of the upper eyelids and an erythematous rash on the face, neck, and anterior chest (V sign) or back and shoulders (shawl sign), knees, elbows, and malleoli. The rash can be pruritic and may worsen after sun exposure. Another characteristic is Gottron papules, a raised violaceous rash or papules at the knuckles, prominent in metacarpophalangeal and interphalangeal joints. This chronic rash can become scaly with a shiny appearance. Dilated fingernail base capillary loops with irregular, thickened, and distorted cuticles can also be seen, and the fingers may appear like so-called mechanic hands, with cracked, horizontal lines that look dirty. Associated proximal muscle weakness can range from mild to severe and may develop before or at the time of the skin changes.
Polymyositis
Polymyositis is another paraneoplastic syndrome associated with lung cancer and presents clinically as a subacute myopathy that evolves over weeks to months, along with weakness of the proximal muscles. Dermatomyositis and polymyositis can develop in different subtypes of lung cancer. It is important to note that these specific paraneoplastic phenomena may be the initial symptoms of lung cancer or may develop during the course of disease progression. Anticancer therapy may help to reduce the symptoms of polymyositis and dermatomyositis; otherwise, corticosteroids are the standard treatment, with immunomodulators offered as an additional therapeutic option.
Endocrine and Metabolic Phenomena
Most endocrine paraneoplastic syndromes result from tumor secretion of peptides or hormones that result in metabolic or homeostatic derangements. Well-known endocrine syndromes associated with lung cancer include syndrome of inappropriate antidiuretic hormone secretion (SIADH), Cushing syndrome, and carcinoid syndrome, as well as metabolic sequelae such as hypercalcemia. Other hormones known to be secreted by lung cancers include interleukin-1α, tumor necrosis factor, human chorionic gonadotropin, transforming growth factor-β, atrial natriuretic peptide, and others. The paraneoplastic endocrine phenomena are typically discovered during initial evaluation of the patient or after diagnosis of lung cancer. These endocrine syndromes may not always correlate with stage or prognosis of the cancer. Anticancer treatments may improve the clinical condition.
Syndrome of Inappropriate Antidiuretic Hormone Secretion
Hyponatremia is a condition associated with many lung diseases, including lung cancer. A study from the 1950s recognized SIADH as a cause of hyponatremia. SIADH is more common in SCLC, affecting 10% to 45% of patients, compared with approximately 1% of patients with other types of cancer. SIADH can cause anorexia, cognition change, confusion, lethargy, and seizures, because antidiuretic hormone secretion leads to persistent renal tubule overexpression of aquaporins and subsequent water resorption. The duration of development and severity of the hyponatremia will influence the clinical symptoms. Life-threatening complications can occur when sodium levels fall to 120 mmol/L or lower, at which point organ failure can occur. The hallmarks of SIADH are euvolemic hyponatremia, plasma hypoosmolality, abnormally high urinary osmolality, and abnormally high urinary sodium concentration in the absence of confounders, such as volume depletion, adrenal insufficiency or hypothyroidism, and medication effects.
The diagnosis of SIADH requires exclusion of other causes, specifically volume depletion, which can confound the diagnostic algorithm and interpretation of laboratory results. The mainstay of SIADH treatment is therapy for the lung cancer, and hyponatremia may resolve within weeks from initiation of chemotherapy for SCLC. In the interval of time before response, the hyponatremia can be managed with fluid restriction, with or without demeclocycline or a vasopressin receptor antagonist (e.g., conivaptan or tolvaptan). Acute severe hyponatremia may be carefully treated with hypertonic saline infusion, but the correction should be gradual so as to avoid overcorrection, thereby minimizing the risk of osmotic demyelination (central pontine demyelination).
Cushing Syndrome
Cushing syndrome has long been recognized as a paraneoplastic phenomenon in cancer, including lung cancer. Between 5% and 10% of Cushing syndrome cases are thought to be paraneoplastic in etiology, and most of these are a result of ectopic adrenocorticotropic hormone (ACTH) secretion rather than corticotropin-releasing hormone. Many cases of Cushing syndrome are related to lung cancer, especially those of neuroendocrine lineage such as SCLCs and carcinoid tumors, which are the lung cancer subtypes most likely to produce ectopic ACTH. The symptoms of ectopic ACTH secretion in lung cancer can vary, and patients may not have all the typical features of Cushing syndrome, given the natural history of lung cancers, especially considering the aggressiveness of SCLC. In SCLC-related Cushing syndrome, there may be aberrant processing of proopiomelanocortin, a precursor of pro-ACTH and ACTH. The precursor levels are elevated more than the ACTH level and correlate with cortisol levels. Conversely, it is thought that bronchial carcinoid tumors process proopiomelanocortin normally and produce more ACTH, reminiscent of pituitary gland overproduction of ACTH. Common symptoms include moon facies and proximal myopathy, as well as hypokalemia and hyperglycemia. Classic features of Cushing syndrome are more likely to occur in bronchial carcinoid tumors.
The diagnosis of Cushing syndrome is confirmed by an elevated cortisol level in a 24-hour urine sample, and an elevated serum ACTH. Failure of suppression by high-dose dexamethasone helps distinguish ectopic paraneoplastic ACTH secretion from pituitary ACTH oversecretion. Imaging studies, including MRI of the pituitary, may be helpful in the differential diagnosis. Treatment should be directed at the cause of the syndrome. When surgical resection of the tumor (i.e., carcinoid) is possible, the syndrome may be resolved. In unresectable lung cancers, medical management includes the use of metyrapone, ketoconazole, somatostatin analogs, aminogluthetimide, tomidate, and mifepristone. If medical management fails, bilateral adrenalectomy may be considered. Cushing syndrome often presents in patients with metastatic disease and portends a poor prognosis.
Carcinoid Syndrome
Carcinoid syndrome, recognized by symptoms of flushing and diarrhea, is the result of secretion of serotonin and other vasoactive substances released into the circulatory system from neuroendocrine tumors. Carcinoid syndrome is mainly associated with metastatic tumors in the midgut, whereas hindgut (distal colorectal) and foregut (gastroduodenal, bronchial) carcinoid tumors are rarely a cause. Nonetheless, approximately 1% to 5% of bronchial neuroendocrine tumors may secrete ectopic serotonin and produce the syndrome. Typical carcinoid syndrome symptoms include flushing of the chest, secretory diarrhea, bronchoconstriction, and, if the syndrome is chronic, it may lead to cardiac valvular fibrosis. Acute episodes may cause cardiovascular collapse and shock. The diagnosis of carcinoid syndrome requires evidence of an abnormal 5-hydroxy-indoleacetic acid level in a 24-hour urine collection, as this is the main metabolite of serotonin (although it may not be of as much value in bronchial carcinoids). In some cases, elevation of the serum chromogranin A levels may also have diagnostic utility, although with low specificity. In terms of imaging, many neuroendocrine tumors express somatostatin receptors, so nuclear octreotide scintigraphy may be considered in addition to conventional imaging. In the future, novel PET isotopes may prove useful for the localization of these tumors. Carcinoid crises, indicated by hypotension, arrhythmias, and bronchospasm, may be precipitated by surgery, anesthesia, biopsy, and drugs such as adrenergic agents or chemotherapy. Acute cases may require early stabilization with octreotide treatment, and, for patients with carcinoid heart disease and severe valvular dysfunction, cardiac surgery may be necessary to improve quality of life and provide survival benefit.
Hypercalcemia
Hypercalcemia often occurs in lung cancer and is usually a result of humoral hypercalcemia of malignancy (HHM) or osteolytic bone metastases. HHM is most commonly associated with squamous cell carcinoma and is caused by the production and secretion of parathyroid hormone (PTH)-related peptide by tumor cells. Its discovery was the culmination of careful research, and most cases of hypercalcemia in lung cancer are now recognized as the result of HHM. The degree of hypercalcemia and the rapidity of biochemical changes influence the presentation. The symptoms of hypercalcemia include cognitive changes, fatigue, polyuria, and abdominal symptoms in conjunction with dehydration. Laboratory tests show hypercalcemia and hypophosphatemia, and electrocardiogram changes may include a prolonged PR or QRS interval, a short QT interval, bradycardia, or heart block. HHM is associated with large tumor burden, male gender, advanced disease, elevated creatinine levels, and poor prognosis. Greater degrees of HHM are associated with the presence of bone metastases, and severe hypercalcemia can lead to coma and death. Diagnosis requires exclusion of other causes, such as metastatic bone involvement, and may be verified by a normal PTH level, low serum phosphorus level, and elevated PTH-related protein level. Management of hypercalcemia involves addressing the calcium levels and associated complications, especially dehydration. Therapeutic strategies include correcting the fluid balance, increasing renal excretion of calcium, and, when possible, reducing bone resorption concurrently with anticancer treatments. Substantial hypercalcemia should be promptly treated; fluid restoration with isotonic saline is beneficial in the renal clearance of calcium, which may be further enhanced by a loop diuretic once adequate hydration has been achieved. Overaggressive rehydration should be avoided because patients with lung cancer may also have coexisting cardiac disease. Bisphosphonates, and particularly other novel agents such as denosumab, are indicated to inhibit calcium release and osteoclast function. Other agents that are effective in treating hypercalcemia include mithramycin, plicamycin, and calcitonin; gallium nitrate treatment has also been used.
Blood Disorders
Anemia
Anemia is often found in patients presenting with lung cancer, and the disorder contributes to symptoms of fatigue and dyspnea. It can be considered a form of anemia of chronic disease if the ferritin levels are either normal or elevated. Other, less common hematologic disorders such as microangiopathic hemolytic anemia, characterized by Coombs-negative, hemolytic anemia with schistocytes and thrombocytopenia, have been reported.
Leukocytosis
Mild leukocytosis is relatively common in lung cancer; however, extreme elevations are rare. Autonomous production of cytokines (granulocyte colony-stimulating factor and granulocyte macrophage colony-stimulating factor) has been noted in some patients with lung cancer, and leukocytosis appears to have a negative prognostic effect. Conversely, hypereosinophilia is not often found in patients with lung cancer, potentially because of tumor overexpression of granulocyte macrophage colony-stimulating factor leading to a leukemoid reaction.
Thrombocytosis
Reactive thrombocytosis is relatively common in cancers, including lung cancer. The prevalence of thrombocytosis in lung cancer is variable, and it is thought to be an independent prognostic factor of survival in patients with primary lung cancer, including operable NSCLC.
Hypercoagulation States
The relationship between cancer and coagulopathy was suggested by Trousseau nearly 150 years ago, and it is now definitively known that patients with lung cancer are at a higher risk of thromboembolic events compared with people who have other types of cancer or who do not have cancer. Several hypercoagulative disorders have been associated with lung cancer, and they vary in severity. These disorders may either be present before the diagnosis of lung cancer or may occur during the course of treatment for the disease. The classic Trousseau syndrome (migratory superficial thrombophlebitis and occasionally arterial emboli) has been noted in patients with lung cancer. Multiple aspects in lung cancer are associated with a higher risk of thrombosis because of patient-related, cancer-related, and treatment-related factors that combined can contribute to the risk of developing a thrombotic event. Tissue factor overexpression is considered by some to be a major contributor to cancer-related thrombosis.
Deep Venous Thrombosis and Thromboembolism
The authors of a large study published in 2008 reported that venous thromboembolism (VTE) developed in approximately 3% of patients with lung cancer within 2 years, and that the incidence of VTE was associated with a higher risk of death within 2 years after the diagnosis of NSCLC or SCLC. Conventional anticoagulation may not be as effective for patients with lung cancer because VTE is more likely to recur in these patients compared with patients without cancer. In a 2011 Cochrane review of randomized clinical trials comparing low-molecular-weight heparin, unfractionated heparin, and fondaparinux (a selective inhibitor of factor Xa) in patients with cancer and objectively confirmed VTE, low-molecular-weight heparin was potentially superior to unfractionated heparin in the initial treatment of VTE in patients with cancer. A paucity of data are available on the ideal duration of anticoagulation therapy for this paraneoplastic cancer syndrome. A systematic review published in 2011 concluded that metastatic malignancy, adenocarcinoma, or lung cancer confers a higher risk of VTE recurrence than localized malignancy or some other cancers. The development of VTE during lung cancer treatment is also not uncommon, and clinicians should be aware of the increased risk and introduce effective preventive measures when indicated. Early data also suggest a possible link between KRAS gene mutations and an increased risk of VTE in NSCLC.
Disseminated Intravascular Coagulopathy
Lung cancer may also be accompanied by disseminated intravascular coagulation, with bone marrow involvement and reduced platelet counts. In addition, idiopathic thrombocytopenic purpura-like syndrome has been reported in patients with NSCLC.
Thrombotic Microangiopathy
Thrombotic thrombocytopenic purpura, a disseminated form of thrombotic microangiopathy, has been found in patients with lung cancer. Immunohistochemical (IHC) staining of lung tumor cells from patients with thrombotic thrombocytopenic purpura has demonstrated endothelial proliferative factors such as vascular endothelial growth factor and osteopontin.
Paraneoplastic Neurologic Syndromes
Paraneoplastic neurologic syndromes (PNSs) are rare, affecting approximately 0.01% of patients with cancer overall, but they are more frequently found in patients with SCLC (3–5%). In some cases, PNS may result from immune crossreactivity between cancer cells and antigens of the nervous system. Antibodies to neuronal surface antigens and intracellular antigens have been reported, and T cells are implicated. Because the tumor cells do not directly produce the syndrome, treatment of the primary cancer may not always abolish the syndrome and additional immunosuppressive therapy is often required. PNS may often be identified before the cancer is diagnosed, and thus evaluation by CT and PET imaging may be necessary. Repeated diagnostic evaluations for cancer may be needed when initial cancer screening does not identify an obvious tumor. The symptoms of PNS depend on the type of neuronal cells affected, ranging from the central nervous system to the peripheral nerves, as well as involvement of neuromuscular junctions.
Central Nervous System
Limbic Encephalitis
As a result of limbic system involvement, manifestations of limbic encephalitis include subacute seizures, memory loss, confusion, and psychiatric symptoms. SCLC is most often associated with limbic encephalitis, but NSCLC has also been linked with the syndrome. A number of neuronal antibodies have been reported in limbic encephalitis, including voltage-gated potassium channel antibodies, GABAb, and others.
Subacute Cerebellar Degeneration
Subacute cerebellar degeneration presents with rapidly developing cerebellar symptoms such as ataxia, nystagmus, and dysarthria. The prognosis for patients with subacute cerebellar degeneration is often poor, because the syndrome is associated with severe disability and impairment. Selective loss of Purkinje fibers is causative, and cerebellar atrophy should be excluded. Associated antibodies in SCLC include anti-Hu (also called anti-neuronal nuclear antibody, type 1 or ANNA-1), anti-Ri, and anti-P/Q voltage-gated calcium channels (VGCCs).
Encephalomyelitis
Encephalomyelitis is characterized by simultaneous dysfunction at various levels of the central nervous system such as the hippocampus, spinal cord, and dorsal root ganglia of myenteric plexus. This condition is mostly found in patients with SCLC. The antibodies implicated include anti-Hu and anti-CV2.
Peripheral Nervous System
Sensory Neuropathy
Sensory neuropathy from damage to the cells of the dorsal root ganglia manifests as subacute onset of asymmetrical numbness, pain, and involvement of the arms and lower limbs as well as proprioceptive loss. Loss of deep tendon reflexes and panmodality sensory loss will be noted on physical examination. The diagnosis is supported by electrophysiologic demonstration of involvement of the sensory fibers. The commonly implicated antibodies in sensory neuronopathy include anti-Hu and anti-CV2, and SCLC is most often associated with this condition.
Autonomic Neuropathy
Autonomic neuropathy may be subacute over weeks and involves the sympathetic, parasympathetic, and enteric systems, resulting in orthostatic hypotension, gastrointestinal tract dysfunction, sicca, bladder and bowel dysfunction, altered pupillary reflexes, loss of sinus arrhythmia, and weight loss. Anti-Hu, anti-CV2, anti-nAchR, and anti-amphiphysin antibodies may be involved.
Neuromuscular Group of Peripheral Nervous System
Lambert-Eaton Myasthenic Syndrome
Lambert-Eaton myasthenic syndrome (LEMS) occurs with proximal muscle weakness, especially at the hip, with progression in cranio-caudal direction. Patients may also have an associated loss of deep tendon reflexes and autonomic dysfunction. The muscle weakness is thought to be caused by damage to VGCCs present on the presynaptic nerve terminal. These same VGCCs are expressed by SCLC, and LEMS may affect up to 3% of patients with SCLC. It is interesting to note that in SCLC LEMS may precede the clinical or radiographic diagnosis of the cancer. Electromyography is helpful and shows low-voltage muscle action potential amplitude and decremental response with low-rate stimulation, but incremental response to high-rate stimulation. LEMS should respond to treatment of the underlying lung cancer, and resistant LEMS may respond to plasma exchange, gamma globulin, and immunosuppression with azathioprine and corticosteroids.
Less Common Peripheral Nervous Systems
Opsoclonus–Myoclonus
Opsoclonus–myoclonus is associated with anti-Ri antibodies in SCLC. Clinical features of the syndrome include myoclonus, involuntary eye movements, and truncal ataxia. Cerebrospinal fluid examination shows increased protein and mild pleocytosis. Partial or complete resolution may occur with treatment of the underlying SCLC.
Cancer-Associated Retinopathy
Cancer-associated retinopathy in SCLC is thought to be caused by damage to the retinal photoreceptors, resulting in scotomas, photosensitivity, and reduced retinal arteriole caliber. Leaks from retinal vessels can be seen with angiography. Spectral-domain optical coherence tomography has also been reported to be useful in the diagnosis of cancer-associated retinopathy. Progression to blindness is common and is caused by an autoantibody to the 23 kDa photoreceptor protein, recoverin. The condition may improve with corticosteroids and anticancer therapy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree