(1)
Department of Surgery, Dar A lAlafia Medical Company, Qatif, Saudi Arabia
4.1 Introduction
Sickle cell anemia (SCA) is an autosomal recessive disorder that results from an abnormal gene found on the short arm of chromosome 11. This leads to the production of a mutated form of hemoglobin, hemoglobin S (HbS), where a single amino acid, glutamic acid, is replaced by valine at the 6th position of the 146 amino acids of hemoglobin beta chain.
A single change of one amino acid causes significant morbidity and mortality (Fig. 4.1).
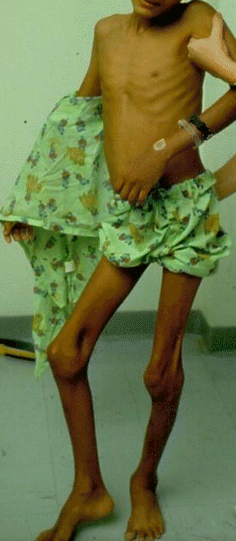
Fig. 4.1
A clinical photograph showing a child with severe sickle cell anemia
Approximately 50 % of patients with SCA develop vaso-occlusive crises. The frequency of these crises is however extremely variable. Some patients have as many as six or more attacks of vaso-occlusive crises per year, whereas others may have episodes only at great intervals or none at all.
SCA usually manifests early in childhood. For the first 6 months of life, infants with sickle cell anemia are protected largely by elevated levels of HbF. Once the level of HbF level decreases, the disease becomes evident.
Early diagnosis, close follow-up, and improved management and supportive care are important in reducing both morbidity and mortality of patients with sickle cell anemia.
As a result of this, screening for sickle cell anemia at birth should be mandatory in areas where sickle cell is common.
4.2 Epidemiology
Sickle cell anemia affects millions of people worldwide in Africa; Greece, Turkey, and Italy; the Arabian Peninsula; India; and Spanish-speaking regions in South America, Central America, and parts of the Caribbean.
The highest frequency of sickle cell anemia is found in tropical regions, particularly sub-Saharan Africa, tribal regions of India, and the Middle East.
Sickle cell anemia occurs more commonly among people whose ancestors lived in tropical and sub-tropical sub-Saharan regions where malaria is or was common.
Patients with sickle cell trait (heterozygous AS) living in these regions have a protective effect, and they develop less severe symptoms when compared with those with sickle cell anemia (homozygous SS).
Around 2 % of newborns in Nigeria were affected by sickle cell anemia, giving a total of 150,000 affected children born every year in Nigeria alone.
The carrier frequency ranges between 10 % and 40 % across equatorial Africa, decreasing to 1–2 % on the North African coast and <1 % in South Africa.
Sickle cell anemia is the most common inherited blood disorder in the United States, affecting 70,000–80,000 Americans. The prevalence of sickle cell anemia in the United States is approximately 1 in 5000 African-American children and one in every 36,000 Hispanic-American children. Sickle cell trait on the other hand occurs among about 1:12 African-Americans and 1:100 Hispanic-Americans.
In France, sickle cell anemia has become the most common genetic disease in the country, with an overall birth prevalence of 1/2,415 in mainland France.
In the United Kingdom, it is estimated that between 12,000 and 15,000 people have sickle cell anemia with an estimate of 250,000 carriers of the gene in England alone.
In Saudi Arabia about 4.2 % of the population carries the sickle cell trait and 0.26 % has sickle cell disease. The highest prevalence is in the Eastern province where approximately 17 % of the population carries the gene and 1.2 % has sickle cell disease.
In India, sickle cell anemia is common in the tribal people of central India, where the prevalence has ranged from 9.4 to 22.2 % in endemic areas of Madhya Pradesh, Rajasthan, and Chhattisgarh.
In Jamaica, 10 % of the population carries the sickle cell gene, making it the most prevalent genetic disorder in the country.
4.3 Clinical Manifestations
The signs and symptoms of sickle cell anemia usually begin in early childhood, but the severity of symptoms varies from one patient to another and also in affected members of the same family.
Some people have mild symptoms, while others are frequently hospitalized for more serious complications.
Although the disease can be diagnosed at birth, clinical manifestations usually do not occur before age 6 months, when the level of HbF decreases and functional asplenia develops.
Functional asplenia results in susceptibility to overwhelming infection with encapsulated bacteria. Patients with sickle cell anemia and as a result of repeated attacks of splenic vaso-occlusive crisis develop splenic infarcts. These repeated splenic infarcts lead to the development of autosplenectomy.
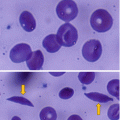
Anemia is a common manifestation of sickle cell anemia and results from premature rupture of red blood cells. Depending on the severity of anemia, it can cause shortness of breath, fatigue, and delayed growth and development in children.
The rapid breakdown of red blood cells may also cause jaundice.
Painful episodes (painful vaso-occlusive crisis) occur in patients with sickle anemia, and these are one of the causes of frequent emergency room visits and hospital admissions of patients with sickle cell anemia.
These attacks are secondary to occlusion of small blood vessels by sickled red blood cells.
A serious complication of sickle cell anemia is pulmonary hypertension.
Affected patients with sickle cell anemia present with a wide range of clinical manifestations that result from vascular obstruction and ischemia.
Hemolysis is a constant feature of patients with sickle cell anemia.
Approximately one-third of RBCs undergo intravascular hemolysis, possibly due to loss of membrane filaments during oxygenation and deoxygenation.
The remainder gets hemolyzed as a result of erythrophagocytosis by macrophages.
Currently, the diagnosis of sickle cell anemia is made early as a result of neonatal screening, but the symptoms usually do not develop until the age of 6–12 months.
This is attributed to the high levels of circulating fetal hemoglobin which exerts a protective effect in these patients.
Subsequently, the clinical manifestations of sickle cell anemia are attributed to:
Hemolytic anemia
Painful vaso-occlusive crisis
Multi-organ damage from sickle cell anemia-related complications
Sickle cell anemia affects every organ in the body and results in several complications.
The clinical severity, however, widely varies, and patients may exhibit some or all of the symptoms described.
Hemolytic anemia:
Sickle cell anemia patients suffer from chronic mild to severe anemia.
This is secondary to repeated hemolysis and phagocytosis of sickled RBCs.
Normal red blood cells last about 120 days, but sickled cells expire after an average of 16 days. This rapid deterioration of red blood cells contributes to anemia.
Repeated episodes of deoxygenation and sickling of RBCs lead to irreversible damage of red blood cell membranes and their hemolysis.
The bone marrow tries to compensate by increasing red blood cell production but is unable to compensate for the high rate of hemolysis. This will lead to production of premature RBCs (reticulocytosis).
Patients with chronic anemia readily adjust and may exhibit only few symptoms of anemia on exertion.
Patients with sickle cell anemia can also develop acute anemic episodes secondary to:
Aplastic crisis
Acute splenic sequestration crisis
Hyperhemolysis
Delayed transfusion reactions from alloantibodies
Hepatic sequestration crisis
Aplastic crisis:
This is seen following infection with parvovirus B-19.
This infection normally causes a benign childhood disorder associated with fever, malaise, anemia, and a mild rash.
This however is not the case in patients with sickle cell anemia.
The virus has trophism for erythroid progenitor cells and impairs cell division for a few days during the infection. This will lead to a shutdown in erythroid production for a few days, and because of the continuous hemolysis, there will be a marked drop in hemoglobin and hematocrit leading to severe anemia.
In these patients and in spite of the severe anemia, there is no reticulocytosis.
Aplastic anemia usually coincides with painful crises.
Treatment of aplastic anemia is supportive, with blood transfusions to maintain an acceptable hematocrit until marrow activity is restored.
Causes of Anemia in Patients with Sickle Cell Anemia
Hemolytic anemia (acute and chronic)
Aplastic crisis
Acute splenic sequestration crisis
Hyperhemolysis
Delayed transfusion reactions from alloantibodies
Hepatic sequestration crisis
Severe infection and multi-organ damage
Splenic complications:
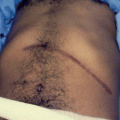
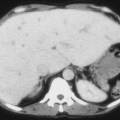
The spleen is normally enlarged during the first decade of life but does undergo progressive atrophy as a result of repeated vaso-occlusive crisis leading to autosplenectomy (Fig. 4.2).
Persistence of splenomegaly in these patients is known to be associated with complications necessitating splenectomy (Figs. 4.3, 4.4, 4.5 and 4.6).
These complications include:
Persistent splenomegaly with a non-functioning spleen
Acute splenic sequestration crisis (minor and major attacks)
Splenic abscess
Hypersplenism
Massive splenic infarction
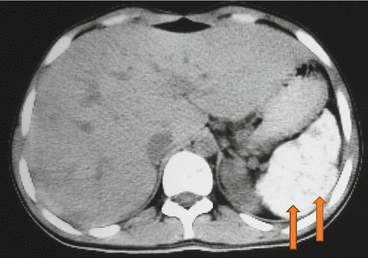
Fig. 4.2
Abdominal CT scan showing an atrophied calcified spleen in a patient with sickle cell anemia
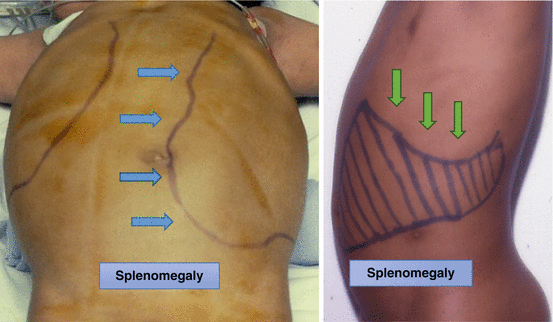
Figs. 4.3 and 4.4
Clinical photographs showing persistent splenomegaly in two patients with sickle cell anemia
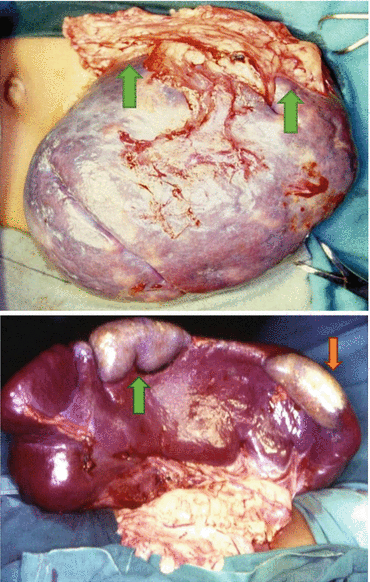
Figs. 4.5 and 4.6
Intraoperative photographs showing enlarged spleens with splenic infarcts. Note the omentum adherent to the splenic infarcts
Splenic Complications of Sickle Cell Anemia
Persistent splenomegaly
Acute splenic sequestration crisis (minor and major attacks)
Splenic abscess
Hypersplenism
Massive splenic infarction
Splenic sequestration crisis:
This occurs with highest frequency during the first 5 years of life in children with sickle cell anemia.
Splenic sequestration crisis can also occur at an older age group in those with persistent splenomegaly.
Acute splenic sequestration crisis and depending on the degree of anemia is divided into major and minor attacks.
Acute splenic sequestration crisis is characterized by:
The onset of life-threatening anemia especially in those with major splenic sequestration crisis where the hemoglobin level can drop to as low as 2 g/dl
Sudden enlargement of the spleen
High reticulocyte count
Regression of the spleen size after blood transfusion
Acute splenic sequestration crisis is a medical emergency and considered the second leading cause of death following infection in children younger than 5 years of age.
To obviate this, it is important to teach the mothers how to palpate the spleen and report to the hospital if there is sudden enlargement of the spleen.
Treatment of acute splenic sequestration crisis requires early recognition, careful monitoring, and aggressive blood transfusion support.
Because these episodes tend to recur, some advocate long-term blood transfusion to prevent recurrence.
Others advocate splenectomy if the child develops one major or two minor sequestration crises.
Characteristics of Acute Splenic Sequestration Crisis
Sudden onset of life-threatening anemia
Sudden enlargement of the spleen
High reticulocyte count
Regression of the spleen size after blood transfusion
Infection:
Children with sickle cell anemia are susceptible to recurrent attacks of infection.
This is because these patients develop functional asplenia early as a result of splenic vaso-occlusive crisis.
Add to this a defective immune response in these patients.
The increased risk of severe bacterial infections is also attributed to loss of functioning spleen as a result of autosplenectomy.
These infections are typically caused by encapsulated organisms such as Streptococcus pneumoniae and Haemophilus influenzae.
To obviate this, daily penicillin prophylaxis is commonly used during childhood.
Add to this the routine vaccination for S. pneumoniae, H. influenzae, and meningococci.
A variety of organisms cause infection in patients with sickle cell anemia, but organisms that pose the greatest danger include Streptococcus pneumonia, Haemophilus influenza, and Salmonella.
The mortality rate of such infections has been reported to be as high as 10–30 %.
Staphylococcus and Salmonella are the two most likely organisms responsible for osteomyelitis and septic arthritis in patients with sickle cell anemia. This is important when considering empirical antibiotic treatment.
Meningitis is 200 times more common in children with sickle cell anemia.
Major Infections in Patients with Sickle Cell Anemia
Septicemia
Pneumonia
Meningitis
Osteomyelitis
Septic arthritis
Splenic abscess
Treatment of infections in patients with sickle cell anemia depends on the site of infection and the likely organisms. Treatment includes:
Early recognition.
Aggressive diagnostic evaluation including CBC count, urinalysis, chest radiographs, and blood cultures.
Prompt administration of intravenous antibiotics. The antibiotic cover should be broad to cover S. pneumonia and other organisms.
Close observation.
Children younger than 12 months with a temperature higher than 39°C who appear toxic, with an infiltrate on chest radiograph and an elevated WBC count, should be admitted to the hospital.
Empirical antibiotics should be started early and should cover all possible organisms depending on the site of infection.
Causative Organisms of Major Infections in Patients with Sickle Cell Anemia
Streptococcus pneumoniae
Haemophilus influenzae
Meningococci
Staphylococcus
Salmonella
Acute chest syndrome:
Acute chest syndrome is a serious complication of sickle cell anemia and one of the causes of mortality (Figs. 4.7 and 4.8).
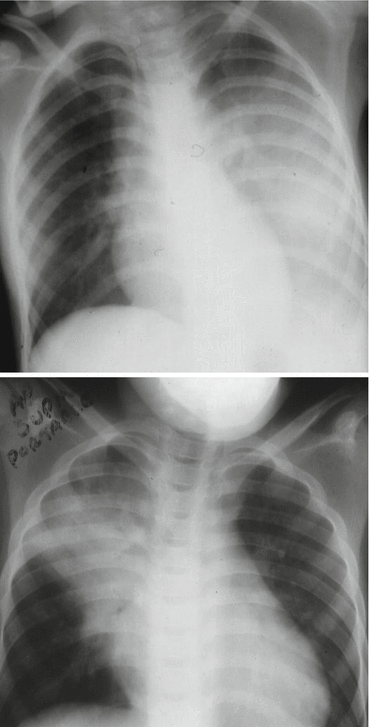
Figs. 4.7 and 4.8
Chest X-rays showing acute chest syndrome. Note the extent of the acute chest syndrome in the first and second chest X-ray
It is defined as a combination of respiratory symptoms, new lung infiltrates on chest X-ray, and fever.
The appearance of the lung infiltrate may be delayed and these patients should be followed up closely.
The lung infiltrate in patients with acute chest syndrome is usually progressive despite treatment with antibiotics.
Children have a higher incidence of acute chest syndrome but a lower mortality rate than adults.
In children, acute chest syndrome is usually due to infection. Other etiologies include pulmonary infarction and secondary fat embolism due to sickling with bone infarction.
Treatment of acute chest syndrome includes:
Close monitoring for hypoxemia.
Oxygen therapy.
Empiric treatment with intravenous antibiotics after appropriate cultures are obtained.
The antibiotics should be active against S. pneumoniae and chlamydial and mycoplasmal organisms.
The antibiotics are changed based on response to therapy and results of cultures and sensitivities.
Patients with moderate or severe acute chest syndrome should be admitted to ICU for close monitoring and observation.
Analgesics including acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs) can be used. Narcotic agents may be used for more severe pain.
Simple packed RBC transfusion can be used, and this may help prevent the progress of acute chest syndrome and respiratory deterioration.
Exchange blood transfusion or erythrocytapheresis can be used in the more severe cases.
Careful intravenous hydration to avoid volume overload, which may contribute to pulmonary infiltrates and exacerbate hypoxia.
The role of corticosteroids in nonasthmatic patients with acute chest syndrome remains controversial.
Respiratory decompensation can develop quickly in these patients and this requires mechanical ventilation.
Acute chest syndrome is known to be recurrent, and one way to prevent this is to give chronic blood transfusion therapy to patients with recurrent acute chest syndrome.
Stroke:
This results from progressive narrowing and occlusion of intracranial blood vessels.
This may lead to cerebral infarction in children and cerebral hemorrhage in adults.
Silent stroke also occur in patients with sickle cell anemia and usually causes no immediate symptoms but can cause brain damage.
Silent stroke is probably five times as common as symptomatic stroke.
About 10–15 % of children with SCA suffer strokes.
Silent strokes predominantly occur in younger patients.
Cholelithiasis and biliary sludge (Figs. 4.9, 4.10, and 4.11):
Cholelithiasis results from excessive bilirubin production and precipitation due to prolonged hemolysis.
Cholelithiasis is common in patients with sickle cell anemia.
The frequency of cholelithiasis is variable but increases with age.
An overall of 19.7 % frequency of gallstones was reported in children with SCA. This frequency increased from 8.7 % in those less than 10 years of age to 36 % in those 15–18 years of age.
It is estimated that an overall 70 % of patients with SCA will develop gallstones at one stage of their life.
Laparoscopic cholecystectomy is advocated for those with cholelithiasis even if they are asymptomatic.
Cholelithiasis can lead to:
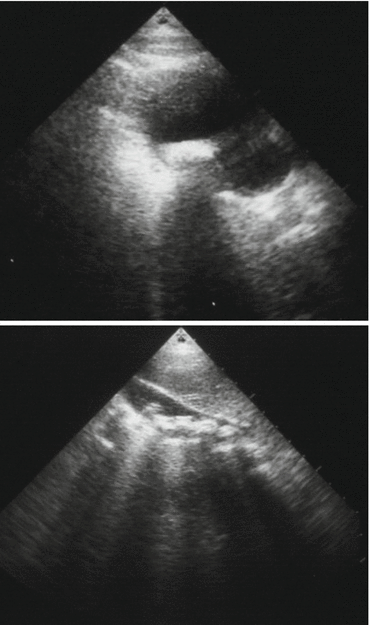
Figs. 4.9 and 4.10
Abdominal ultrasound showing a single and multiple gallstones in patients with sickle cell anemia
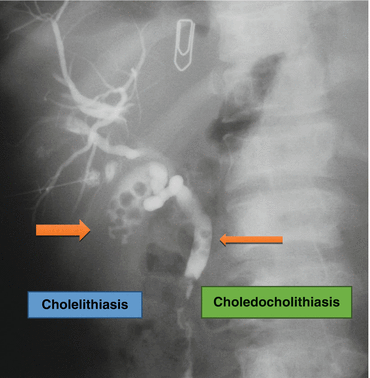
Fig. 4.11
A PTC (percutaneous transhepatic cholangiogram) showing cholelithiasis and choledocholithiasis
Complications of Cholelithiasis
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree