Summary of Key Points
- •
Lung cancer incidence and mortality has declined among men in many countries, following a decline in the prevalence and level of smoking. Among women, lung cancer incidence and mortality is still increasing in many countries and has become the main cause of cancer death.
- •
Despite important advances in lung cancer screening, primary prevention through tobacco control remains the main approach in the fight against lung cancer, especially in low-income countries.
- •
Occupational factors, passive smoking and other indoor pollutants, including radon, and air pollution are other important modifiable causes of lung cancer; nutritional factors and infectious agents are additional potential risk factors. Control of exposure to lung carcinogens other than tobacco, in both the general and the occupational environment, has had a substantial impact in several high-risk populations.
- •
Lung cancer in never-smokers is not an uncommon disease. While there is an interaction between tobacco smoking and other lung carcinogens, several agents have been shown to cause lung cancer also in never-smokers.
- •
Lung cancer was the most important epidemic of the 20th century, and it is likely to remain a major public health problem in the 21st century. It is also a paradigm of the importance of primary prevention and a reminder that scientific knowledge is not sufficient per se to ensure human health.
The history of lung cancer epidemiology parallels the history of modern chronic disease epidemiology. In the 19th century, an excess of lung cancer was observed among miners and some other occupational groups, but otherwise the disease was very rare. An epidemic increase in lung cancer began in the first half of the 20th century, with much speculation and controversy about its possible environmental causes.
Among both women and men, the incidence of lung cancer is low in persons under 40 years of age, it increases up to age 70 or 75 years ( Fig. 1.1 ), and it declines thereafter. The decline in incidence in the older-age groups can be explained, at least in part, by incomplete diagnosis or by a generation (birth cohort) effect.
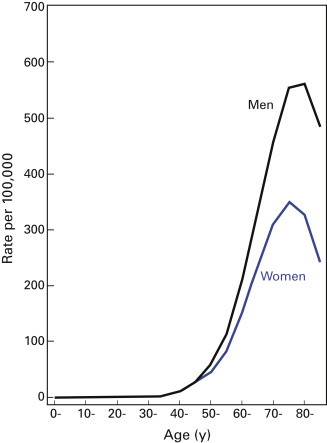
Methodologically, epidemiologic studies of lung cancer have been straightforward because the site of origin is well defined, progressive symptoms prompt diagnostic activity, and the predominant causes are comparatively easy to ascertain. Novel approaches to the classification of lung cancer based on molecular techniques will likely bring new insights into its etiology, especially among nonsmokers.
Descriptive Epidemiology
Lung cancer, a rare disease until the beginning of the 20th century, has become the most frequent malignant neoplasm among men in most countries and the main neoplastic cause of death in both men and women. In 2012, lung cancer accounted for an estimated 1,242,000 new cancer cases among men, which is 17% of all cancers excluding nonmelanoma skin cancer, and 583,000, or 9%, of new cancers among women. After nonmelanocytic skin cancer, lung cancer is the most frequent malignant neoplasm in humans and the most important cause of neoplastic death. Approximately 58% of all cancers occur in developing countries.
The geographic and temporal patterns of lung cancer incidence are determined chiefly by consumption of tobacco. An increase in tobacco consumption is paralleled a few decades later by an increase in the incidence of lung cancer, and a decrease in consumption is followed by a decrease in incidence. Other factors, such as genetic susceptibility, poor diet, and indoor air pollution, may act in concert with tobacco smoking in shaping the descriptive epidemiology of lung cancer.
The pattern found today in men ( Fig. 1.2 ) is composed of populations at high risk, in which consumption of tobacco has been persistently high for decades, and populations at low risk, either because tobacco consumption has not been increasing for long (e.g., China, Africa) or because a decrease in consumption has been present for several decades (e.g., Sweden).
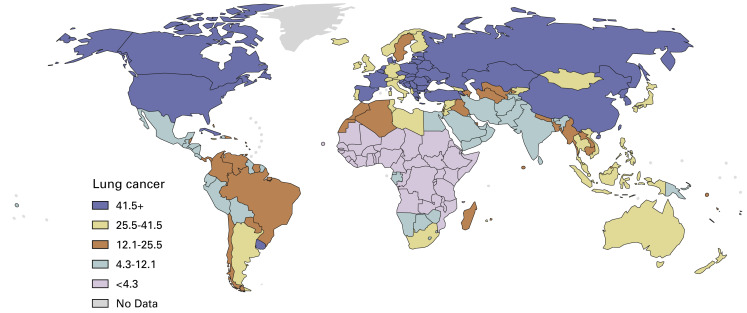
In countries with populations made up of different ethnic groups, differences in lung cancer rates are frequently observed. For example, in the United States, the rates are higher among black men than among other ethnic groups ( Table 1.1 ).
| Ethnic Group | Men | Women |
|---|---|---|
| Asian and Pacific Islander | 31.6 | 17.5 |
| Black | 66.8 | 35.5 |
| Hispanic white | 25.0 | 16.5 |
| Non-Hispanic white | 51.2 | 38.1 |
a Data from the US Surveillance, Epidemiology End-Result database for 2003–2007.
Over the past 25 years, the distribution of histologic types of lung cancer has been changing. In the United States, squamous cell carcinoma, which was formerly the predominant type, is decreasing, whereas adenocarcinoma has increased in both genders. In Europe, similar changes are occurring in men, whereas in women, both squamous cell carcinoma and adenocarcinoma are increasing. Although the increase in the incidence of adenocarcinoma may be due, at least in part, to improved diagnostic techniques, changes in composition and patterns of tobacco consumption (deeper inhalation of low-nicotine and tar tobacco smoke) are additional explanations.
Risk Factors
Tobacco Smoking
The evidence is very strong that tobacco smoking causes all major histologic types of lung cancer. A carcinogenic effect of tobacco smoke on the lung has been demonstrated in epidemiologic studies conducted since the early 1950s and has been recognized by public health and regulatory authorities since the mid-1960s. Tobacco smoking is the main cause of lung cancer in most populations, and the geographic and temporal patterns of the disease largely reflect tobacco consumption during the previous decades. Because of the high carcinogenic potency of tobacco smoke, a major reduction in tobacco consumption would result in the prevention of a large fraction of human cancers.
The excess risk among continuous smokers relative to the risk among never-smokers is on the order of 10-fold to 20-fold. The overall relative risk reflects the contribution of the different aspects of tobacco smoking: average consumption, duration of smoking, time since quitting, age at start, type of tobacco product, and inhalation pattern, as well as the absolute risk in never-smokers.
Several large cohort and case–control studies have provided detailed information on the relative contributions of duration and amount of cigarette smoking to excess lung cancer risk. Doll and Peto analyzed data from a large cohort of British doctors and concluded that the excess lung cancer risk rises in proportion to the square of the number of cigarettes smoked per day but to the fourth power of the duration of smoking. Therefore duration of smoking should be considered the strongest determinant of lung cancer risk in smokers. Analysis of the same cohort after 50 years of follow-up confirmed these results.
An important aspect of tobacco-related lung carcinogenesis is the effect of cessation of smoking. The excess risk sharply decreases in ex-smokers, starting approximately 5 years after quitting, and an effect is apparent even for cessation late in life. However, an excess risk throughout life likely persists even in long-term quitters.
The risk of lung cancer is lower among smokers of low-tar cigarettes than among smokers of high-tar cigarettes and lower among smokers of filtered cigarettes than among smokers of unfiltered cigarettes. Smokers of black (air-cured) tobacco cigarettes are at twofold to threefold higher risk of lung cancer than smokers of blond (flue-cured) tobacco cigarettes. Tar content, the presence or absence of a filter, and the type of tobacco are not independent, however. High-tar cigarettes tend to be unfiltered, and in countries where both black and blond tobacco are used, cigarettes are more frequently made from black tobacco.
Although cigarettes are the main tobacco product smoked in Western countries, an exposure–response relationship with lung cancer risk has also been shown for cigars, cigarillos, and pipes, indicating a carcinogenic effect of these products as well. An increased risk of lung cancer has also been shown after consumption of local tobacco products, such as bidi and hookah in India, khii yoo in Thailand, and water pipe in China. Limited data suggest an increased lung cancer risk after consumption of other tobacco products, such as narghile in western Asia and northern Africa and toombak in Sudan.
Differences in the Effect of Tobacco Smoking According to Histology, Gender, and Race
Although the evidence is abundant that tobacco smoking causes all major histologic types of lung cancer, the associations appear to be stronger for squamous cell and small cell carcinoma and weaker for adenocarcinoma. The incidence of adenocarcinoma has greatly increased during the past decades. Some of the increase may be attributable to improved diagnostic techniques, but aspects of tobacco smoking may also have played a role; it is unclear, however, which aspects of smoking might explain these changes.
A few studies have suggested a difference in the risk of lung cancer between men and women who have smoked a comparable amount of tobacco, but most of the available evidence does not support this gender difference.
The higher rate of lung cancer among the black population compared with the rates in other ethnic groups in the United States is probably explained by the higher tobacco consumption in that population. The lower risk of lung cancer among smokers in China and Japan compared with the risks among smokers in Europe and North America may be due to the relatively recent beginning of regular heavy smoking in Asia, although differences in the composition of traditional smoking products and in genetic susceptibility may also play a role.
Secondhand Tobacco Smoke
The epidemiologic evidence and biologic plausibility support a causal association between secondhand exposure to cigarette smoke and lung cancer risk in nonsmokers. The evidence of a high relative risk in the original studies has been challenged on the basis of both possible confounding by active smoking, diet, or other factors and possible reporting bias. However, when these factors were taken into account, the association was confirmed, and the excess risk was on the order of 20% to 25%.
The effect of involuntary smoking appears to be present for both household exposure, mainly from the spouse, and workplace exposure. By contrast, little evidence has been found for an effect of childhood involuntary smoking exposure.
Confounding Effects of Tobacco Smoking
The importance of tobacco smoking in the causation of lung cancer complicates the investigation of the other causes of this disease because tobacco smoking may act as a powerful confounder. For example, a population of industrial workers exposed to a suspected carcinogen may smoke more than the unexposed comparison population. An excessive lung cancer risk in the exposed group, especially if small, might be due to the difference in smoking rather than to the effect of the occupational agent. One solution is to restrict the investigation to lifetime nonsmokers. However, they may represent a selected group, with low prevalence of exposure to many agents of interest. An alternative is to collect detailed information on smoking habits and to compare the effect of the suspected carcinogens across different groups of smokers. This approach has shown that tobacco smoking as a confounder rarely completely explains excess risks larger than about 50%.
Interaction Between Tobacco Smoke and Other Lung Carcinogens
Other carcinogens may interact with tobacco smoke in the determination of their carcinogenic action on the lung. In other words, the absolute or relative risk from exposure to another agent may be greater (or smaller) among heavy smokers compared with the corresponding risk among light smokers and nonsmokers. The interaction may take place at the stage of exposure; that is, the other agent has to be absorbed on the tobacco particles to penetrate the lung. Or it may take place at some stage of the carcinogenic process, for example, on induction of common metabolic enzymes or activation of common molecular targets. The empirical evidence for an interaction between tobacco smoking and other agents is scanty, mainly because of lack of data among light smokers and nonsmokers. The interaction between asbestos exposure and tobacco smoking falls between the additive and the multiplicative model. The interaction between radon exposure and tobacco smoking best fits a submultiplicative model; data for other agents are too sparse to allow conclusions.
Use of Smokeless Tobacco Products
Few studies have investigated the risk of lung cancer among users of smokeless tobacco products. In two large cohorts of US volunteers, the relative risk of lung cancer associated with spit tobacco use among nonsmokers was 1.08 (95% confidence interval [CI], 0.64–1.83) and 2.00 (95% CI, 1.23–3.24). In a Swedish cohort, the relative risk of lung cancer for every use of snus was 0.80 (95% CI, 0.61–1.05). In a large case–control study from India, the relative risk of lung cancer for every use of tobacco-containing chewing products was 0.74 (95% CI, 0.57–0.96). Overall, the evidence of an increased risk of lung cancer from use of smokeless tobacco products is weak; the apparent protective effect detected in studies including smokers may be due to uncontrolled negative confounding.
Dietary Factors
Vegetables and Fruits
There is some evidence that a diet rich in vegetables and fruits probably exerts a protective effect against lung cancer. Although a protective effect of high vegetable and fruit intake was found in most case–control studies, results of prospective studies with detailed information on dietary intake are less consistent in showing a similar effect. Possible reasons for the inconsistent results include bias from retrospective dietary assessment, misclassification and limited heterogeneity of exposure in cohort studies, residual confounding by smoking, and variability in food composition. Among specific types of fruits and vegetables, the evidence is stronger for cruciferous vegetables, but even in this case it is unlikely that this group of foods represents a strong protective factor against lung cancer.
Meat and Other Foods
It has been suggested that high intake of meat, in particular fried or well-done red meat, increases the risk of lung cancer, although the available evidence does not support this hypothesis. If real, the association may be explained by the formation of nitrosamines during cooking of the meat, as well as by the saturated fat content of meat (as discussed later). Although risk estimates for the intake of other foods, such as cereals, pulses, eggs, milk, and dairy products, have been specified in some studies, these results are inadequate for a judgment of the evidence of an effect.
Coffee and Tea
In a few studies, high consumption of coffee has been associated with an increased risk of lung cancer. However, residual confounding by tobacco smoking is a distinct possibility, and no conclusion can be drawn at present. There is some evidence of a chemopreventive effect of tea, notably green tea, in smokers. The overall evidence, however, is not consistent.
Lipids
In several ecologic studies, a positive association was found between total lipid intake and lung cancer risk that appears to be independent of the risk of tobacco consumption. The analytic studies that have addressed this association, however, have produced mixed results. Although no study has provided evidence of a protective effect of total lipid intake, an increased risk was shown only in case–control studies, whereas a pooled analysis of eight cohort studies provided no evidence of an increased risk of lung cancer for high intake of either total fat or saturated fat.
Carotenoids
Many studies have addressed the risk of lung cancer in relation to estimated intake of either beta-carotene or total carotenoids (which in most cases correspond to the sum of alpha- and beta-carotene). Five cohort and 18 case–control studies published up to 1994 provided 28 risk estimates in different populations; with one notable exception, 25 of these estimates indicated a protective effect of high beta-carotene intake. The protective effect provided a 30% to 80% reduction in the risk of lung cancer between the highest and lowest intake categories. The risk decreased for all major histologic types of lung cancer in many countries, in both genders, and in both smokers and nonsmokers. Similar results have been obtained in studies based on measurement of beta-carotene in prospectively collected sera. The evidence of a protective effect from most observational studies has been refuted by the results of randomized intervention trials based on beta-carotene supplementation ( Table 1.2 ). In two of these trials, which included smokers or workers exposed to asbestos, a significant increase in the incidence of lung cancer was observed in the treated groups; in the remaining studies, no effect was ascertained. The difference in results between observational studies and preventive trials can be explained by confounding by cancer-protective factors in fruits and vegetables other than beta-carotene or by the possibility that high, nonphysiologic doses of beta-carotene may cause oxidative damage, especially among smokers.
| Author | Setting, Population, Age (y) | Follow-up | Daily Dose (mg) | RR | 95% CI |
|---|---|---|---|---|---|
| Kamangar et al. (2006) | Linxian (China), 29,584, 40–69 | 1986–2001 | 15 a | 0.98 | 0.71–1.35 |
| ATBCCP Study Group (1994) | Finland, 29,133 male smokers, 50–69 | 1985–1993 b | 20 | 1.18 | 1.03–1.36 |
| Hennekens et al. (1996) | United States, 22,071 male physicians, 40–84 | 1982–1995 | 25 c | 0.93 | NA |
| Omenn et al. (1994) | United States, 18,314 smokers or asbestos workers, 45–74 | 1985–1995 | 30 | 1.28 | 1.04–1.57 |
a Combined with selenium (50 μg) and alpha-tocopherol (30 mg).
b Follow-up for cancer incidence.
Other Micronutrients
For none of the antioxidant vitamins or the other micronutrients is there conclusive evidence of a protective effect against lung cancer. The data for selenium, vitamin A, lutein, and lycopene, in particular, are inconclusive. The results of studies of serum level of these micronutrients are insufficient for an evaluation. There is evidence from observational studies that low levels of vitamin D are associated with lung cancer risk; results of randomized trials, however, do not provide supportive evidence, arguing for caution in drawing conclusions.
Isothiocyanates
Isothiocyanates are a group of chemicals with cancer-preventive activity in experimental systems and may be responsible for the possibly reduced risk of lung cancer associated with high intake of cruciferous vegetables. The enzymes glutathione S-transferase M1 and T1 are involved in their metabolism. As indicated, these enzymes are polymorphic, with 5% to 10% of Europeans and 30% to 40% of Asians being carriers of a deletion in both. In four studies it has been shown that the protective effect of a high intake of isothiocyanates is stronger in carriers of both deletions than in other noncarriers ( Fig. 1.3 ). No final conclusions can be drawn, but this effect is an example of a possible gene–environment interaction in lung carcinogenesis.
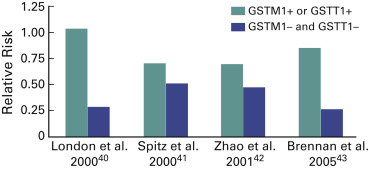
Alcohol
Given the strong correlation between alcohol drinking and tobacco smoking in many populations, it is difficult to disentangle the contribution of alcohol to lung carcinogenesis while properly controlling for the potential confounding effect of tobacco. Meta-analyses have demonstrated that the increased risk of lung cancer observed among alcoholics is mainly attributable to such residual confounding, but some evidence of a smoking-adjusted association with high alcohol consumption was found. This conclusion was confirmed by a pooled analysis of seven cohort studies. Overall, it may be premature to conclude that an association between alcohol drinking and lung cancer has been confirmed by the available data. If the association is causal, alcohol may act as a solvent for carcinogens such as the ones in tobacco smoke. In addition, alcohol can induce metabolic enzymes or act through direct DNA damage via the active metabolite acetaldehyde.
Hormones
Estrogen and progesterone receptors are expressed in the normal lung and in lung cancer cell lines, and estradiol has a proliferative effect on lung cancer cells. Although an effect of estrogens on lung carcinogenesis has not been demonstrated, estrogens may act via formation of DNA adducts and activation of growth factors. Data on risk of lung cancer after the use of hormone replacement therapy have been reported from five case–control studies, two cohort studies, and one randomized trial. A small increased risk of lung cancer has been found in the early studies, whereas a decreased risk was detected in the more recent studies. No effect was observed in the only randomized trial. Although the different results may be explained by changes in the formulations used for replacement therapy, the lack of an effect in the only study with an experimental design argues against an effect of this type of exposure on lung cancer.
Three cohort studies and one case–control study were included in a meta-analysis of serum insulin-like growth factor 1 level and lung cancer. The overall relative risk was 1.01 (95% CI, 0.49–2.11). The results for insulin-like growth factor–binding protein 3 level were also negative (summary relative risk, 0.83; 95% CI, 0.38–1.84), although exclusion of a deviant study resulted in a decreased risk of lung cancer for a high level of insulin-like growth factor–binding protein 3 (relative risk, 0.53; 95% CI, 0.34–0.83).
Anthropometric Measures
There is some evidence for association between a reduced body mass index and an increased risk of lung cancer.
However, this inverse association can be explained, at least in part, by negative confounding by smoking, and no clear association has been demonstrated among never-smokers. Subsequent studies have supported this conclusion that the apparent association is due to confounding.
Evidence suggests a direct association between height and lung cancer risk. Subsequent studies have supported this finding, although the evidence is not fully consistent.
Infections
People with pulmonary tuberculosis have been found to be at increased risk of lung cancer. A similar association was reported from community-based studies among smoking and nonsmoking women. In the most informative study, involving a large cohort of people with tuberculosis from Shanghai, China, the relative risk of lung cancer in the whole cohort was 1.5 and it was 2.0 20 years after the diagnosis of tuberculosis; a correlation was also seen with the location of the tuberculosis lesions. Whether the excess risk is caused by the chronic inflammatory status of the lung parenchyma or by the specific action of the Mycobacterium is not clear. A role of isoniazid, a widely used tuberculosis drug that causes lung tumors in experimental animals, was excluded in one large study.
Chlamydia pneumoniae is a cause of acute respiratory infection. Six studies have been published on the risk of lung cancer among individuals with markers of C. pneumoniae infection. A positive association was detected in all six studies. However, studies based on prediagnostic samples had lower risk estimates than studies based on postdiagnostic samples. An association between infection with human papilloma virus and lung cancer, in particular the adenocarcinoma type, has been suggested by the results of an analysis of series of cases and by the growing evidence of an increased risk among workers potentially exposed to this agent, such as butchers. The results are insufficient to draw a conclusion about the presence or absence of a causal association. Other biologic agents that have been suggested as playing a role in lung carcinogenesis include simian virus 40 and the fungus Microsporum canis .
Ionizing Radiation
There is conclusive evidence that high exposure to ionizing radiation increases the risk of lung cancer. Atomic bomb survivors and patients treated with radiotherapy for ankylosing spondylitis or breast cancer are at moderately increased risk of lung cancer (relative risk, 1.5–2.0 for cumulative exposure in excess of 100 rad). The association with high doses of ionizing radiation was stronger for small cell carcinoma than for other histologic types of lung cancer. Studies of nuclear industry workers exposed to relatively low levels of ionizing radiation, however, provided no evidence of an increased risk of lung cancer.
Underground miners exposed to radioactive radon and its decay products, which emit alpha particles, have been consistently found to be at increased risk of lung cancer. The risk increased with estimated cumulative exposure and decreased with attained age and time since cessation of exposure. In a pooled analysis of 11 cohorts, an apparently linear, approximately 6% risk increase per working-level year of exposure was estimated. Evidence was also found that for comparable cumulative exposure, the risk is greater for lower rates over a longer period and that smoking modifies the carcinogenic effect of radon. Today the main concern about lung cancer risk from radon and its decay products comes from residential rather than occupational exposure. In a pooled analysis of 13 European case–control studies, a relative risk of 1.084 (95% CI, 1.030–1.158) per 100 Bq/m 3 increase in measured indoor radon was found. After correction for the dilution caused by measurement error, the relative risk was 1.16 (95% CI, 1.05–1.31). The exposure–response relationship was linear with no evidence of a threshold. The same conclusion was reached from a similar analysis of North American studies. These results suggest that indoor radon exposure may be an important cause of lung cancer, in particular among nonsmokers unexposed to occupational carcinogens.
Occupational Exposures
The important role of specific occupational exposures in lung cancer etiology has been well established in reports dating back to the 1950s. The risk of lung cancer is increased among workers employed in a number of industries and occupations ( Table 1.3 ). The responsible agents have been identified for several, but not all, of these high-risk workplaces. Evidence for the carcinogenicity of many occupational agents has been reviewed. Estimates of the proportion of lung cancer cases attributable to occupational agents in France (12.5% in men and 6.5% in women) and the United Kingdom (14.5% overall) have been reported in two studies, published in 2010 and 2012, respectively. Although asbestos remains the most important occupational lung carcinogen, the precise role of silica, radon, heavy metals, and polycyclic aromatic hydrocarbons (PAHs) in the burden of occupational cancer is uncertain. The remaining occupational lung carcinogens are likely to play a lesser role in terms of disease burden.