Key words
Anatomic pathology, Cytology sample, Lung cancer classification, Pathologic classification of lung cancer, Pathologic diagnosis, Resected tumor, Small biopsy specimen, Tissue management
Summary of Key Points
- •
A clinically relevant pathologic classification of lung cancer is essential for accurate diagnosis and for patients to receive appropriate therapy.
- •
Although classification of the majority of lung cancers is straightforward, areas of controversy and diagnostic challenges remain.
- •
The current lung cancer classification is applicable to surgically resected tumors and small biopsy specimens.
- •
Pathologists play a critical role in properly handling tissue and cytology specimens for molecular testing of lung cancer.
- •
The recently developed classification and approaches for lung cancer diagnosis are aligned with current clinical practice and open new avenues for research.
Lung cancer continues to be the most common and deadly malignancy worldwide. The main challenge to improve the poor survival rate (5-year survival approximately 15%) of this disease is to develop better strategies for stratifying high-risk populations for early diagnosis and for selecting adequate treatment for different subsets of lung cancer. The mortality associated with this disease is high primarily because most lung cancers are diagnosed at advanced stages, when options for treatment are mostly palliative. Accurate pathologic classification and diagnosis of lung cancer are essential for patients to receive appropriate therapy. Although classification of the vast majority of lung cancers is straightforward, areas of controversy and diagnostic challenges remain.
From pathologic and biologic perspectives, lung cancer is a highly complex neoplasm with several histologic types. Although most lung cancers are associated with smoking, a significant proportion of them (approximately 15%) occur among never-smokers, mostly with adenocarcinoma tumor histology. Lung tumors are the result of a multistep process in which normal lung cells accumulate multiple genetic and epigenetic abnormalities and evolve into cells with malignant biologic capabilities. Recent advances in understanding the complex biology of nonsmall cell lung carcinoma (NSCLC), particularly activation of oncogenes by mutation, translocation, and amplification, have provided new treatment targets and allowed the identification of subsets of NSCLC tumors with unique molecular profiles that can predict response to therapy in this disease. The identification of a specific genetic and molecular abnormality using tumor tissue specimens, followed by administration of a specific inhibitor to the target, is the basis of personalized cancer treatment. In this new paradigm for lung cancer, making a precise pathologic diagnosis and properly handling tissue and cytology samples for molecular testing are becoming increasingly important. These changes in the paradigms of lung cancer diagnosis and treatment have posed multiple new challenges for pathologists to adequately integrate both routine histopathologic analysis and molecular testing into the clinical examination for tumor diagnosis and subsequent selection of the most appropriate therapy.
In this chapter we describe the pathologic features of the major types of lung cancer and the diagnostic tools available for their diagnosis, with special emphasis on the challenges involved in classifying lung cancer using small tissue biopsies and cytology specimens. In Table 17.1 we summarize the current approach for the histologic classification of surgically resected tumors in lung cancer as described in the new lung cancer classifications from the World Health Organization.
| Category | Description |
|---|---|
| Adenocarcinoma |
|
| Squamous cell carcinoma |
|
| Large cell carcinoma | |
| Neuroendocrine tumors |
|
| Adenosquamous carcinoma | |
| Sarcomatoid carcinoma |
|
| Other and unclassified carcinomas |
|
| Salivary gland tumors |
|
| Papillomas |
|
| Adenomas |
|
| Mesenchymal tumors | |
| Lymphohistiocytic tumors | |
| Tumors of ectopic origin Metastatic tumors | |
Nonsmall Cell Lung Carcinoma
The generally denominated NSCLCs are by far the most common type (representing approximately 85%) of lung cancer. Although NSCLC displays numerous histologic patterns, most tumors can be grouped into three main categories: squamous cell carcinoma (30%), adenocarcinoma (40%), and large cell carcinoma (3–9%). Traditionally, the NSCLC designation was used for tumors that had histologic and cytologic features different than small cell carcinoma (SCLC). However, more recently, with the use of new therapeutic strategies and molecular diagnostic testing, it has become imperative to provide a more specific diagnosis of lung cancer, and the histologic subtypes must be part of the pathology report. As explained later, pathologists are more often required to perform a panel of immunohistochemical staining in biopsy and cytology specimens when the histologic subtype is not clear.
Adenocarcinoma
Adenocarcinoma is a malignant epithelial tumor with glandular differentiation or mucin production, showing various growth patterns, with expression of either mucin or thyroid transcription factor-1 (TTF-1). A significant change in pathologic classification of lung cancer occurred in 2011 with the publication of the revised classification of lung adenocarcinoma under the sponsorship of the International Association for the Study of Lung Cancer (IASLC), the American Thoracic Society (ATS), and the European Respiratory Society (ERS). This new classification of adenocarcinoma outlined many paradigm shifts that affect clinical diagnosis and management and open new avenues for research. A major point in this classification was the concept that personalized medicine for patients with advanced lung cancer is determined by histology and genetics and that strategic tissue management of small biopsy specimens is critical for pathologic and molecular diagnosis. This publication was a multidisciplinary effort rather than one primarily addressed by pathologists; clinicians, radiologists, molecular biologists, and surgeons were involved. This collaboration led to an emphasis on correlations between pathology and clinical, radiologic, and molecular characteristics. In addition, the experts recognized that 70% of patients with lung cancer present with advanced-stage disease, which is usually diagnosed based on small biopsy and cytology specimens. Because the previous (2004) classifications from the WHO focused on lung cancer diagnosis in resection specimens, which are obtained in only 30% of cases, a major new effort was made in this new classification to define terminology and criteria to be used in small biopsies and cytology specimens. Therefore, this classification is divided into two sections based on the diagnostic modalities of lung cancer ( Table 17.2 ). These changes have been reflected in the recently released 2015 WHO Classification of Lung Cancer. Resection specimens apply for patients with early-stage disease who are eligible for surgical resection, and small biopsy and cytology specimens for patients with advanced-stage lung cancer.
| Category | Description |
|---|---|
| Preinvasive lesions |
|
| Minimally invasive adenocarcinoma (≤3-cm lepidic predominant tumor with ≤5-mm invasion) |
|
| Invasive adenocarcinoma |
|
| Variants of invasive adenocarcinoma |
|
a Classification of the International Association for the Study of Lung Cancer, American Thoracic Society, and European Respiratory Society (IASLC/ATS/ERS).
Several important modifications were made to the 2004 WHO classification concerning specimens in the 2011 IASLC/ATS/ERS and 2015 WHO classifications. The most significant change is the discontinuation of the term bronchiolo–alveolar cell carcinoma (BAC). This term had been used for at least five different entities with disparate clinical and molecular properties, leading to great confusion in routine clinical care and research. To address two of these entities, the concepts of adenocarcinoma in situ (AIS) and minimally invasive adenocarcinoma (MIA) were proposed for small (no more than 3 cm), solitary adenocarcinomas with a lepidic pattern that either lack invasion (AIS) or only show small foci of invasion measuring no more than 0.5 cm (MIA). AIS and MIA should define patients with either 100% or near 100% 5-year disease-free survival if the tumor is completely resected. The term mixed subtype was discontinued, and invasive adenocarcinomas were classified according to their predominant subtype. Using this approach, the proportions of each of the histologic subtypes should be estimated in a semiquantitative manner and a predominant pattern designated. The term lepidic predominant adenocarcinoma was proposed for nonmucinous tumors classified formerly as mixed subtype where the predominant subtype consists of the former nonmucinous BAC. Micropapillary adenocarcinoma was introduced as a major histologic subtype as multiple studies have shown that patients with such tumors have a poor prognosis. The tumors formerly classified as mucinous BAC are now reclassified as mucinous AIS or MIA or invasive mucinous adenocarcinoma; these tumors on computed tomography (CT) scan frequently show nodules of consolidation with air bronchograms and a multinodular and multilobar distribution. Finally, clear cell and signet ring adenocarcinomas were discontinued as major subtypes because they represent cytologic features that can occur in multiple histologic patterns of adenocarcinoma; however, now these features can be recorded when any amount is present.
In the new classification, tumors formerly regarded as BAC included a wide spectrum of entities with varied clinical behavior such as AIS, MIA, lepidic predominant adenocarcinoma, overtly invasive adenocarcinoma with a lepidic component, and invasive mucinous adenocarcinoma. AIS should not be equated with tumors previously classified as BAC, particularly in registry databases such as Surveillance, Epidemiology, and End Results Program (SEER). Such data could be misleading as AIS is the rarest lung adenocarcinoma, representing only 0.2% to 3% of cases in white populations and up to 5% in a Japanese series. Most cases previously classified as BAC represent tumors with invasive components. Since the publication of the 2011 IASLC/ETS/ERS classification, a series of studies validated various aspects of the classification in resection specimens. Studies from Australia, Europe, Asia, and North America have demonstrated that the proposed subtyping has prognostic value.
Atypical Adenomatous Hyperplasia
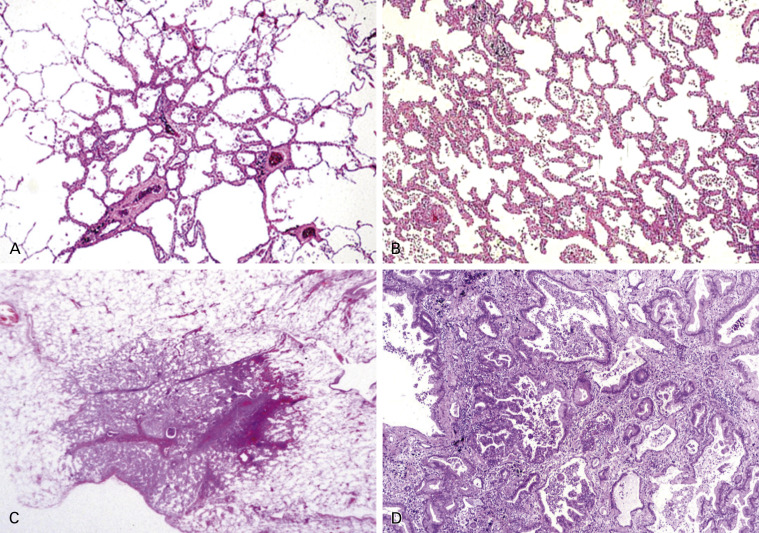
Atypical adenomatous hyperplasia (AAH) is considered to be a precursor of adenocarcinoma. AAH is a discrete parenchymal lesion in the alveoli close to terminal and respiratory bronchioles. Because of their size, AAH cells are usually incidental histologic findings, but they may be detected grossly, especially if they are 0.5 cm or larger. The increasing use of high-resolution CT scans for screening purposes has led to an increasing awareness of AAH, which remains one of the most important differential diagnoses of air-filled peripheral lesions (so-called ground-glass opacities). AAH maintains an alveolar structure lined by rounded, cuboidal, or low columnar cells ( Fig. 17.1A ). The postulated progression of AAH to adenocarcinoma with predominant lepidic growth features, apparent from the increasingly atypical morphology, is supported by the results of morphometric, cytofluorometric, and molecular studies. The origin of AAH is still unknown, but the differentiation phenotype derived from immunohistochemical and ultrastructural features suggests an origin from the progenitor cells of the peripheral airways, such Clara cells and type II pneumocytes.
Adenocarcinoma in Situ
AIS was added to the group of preinvasive lesions along with AAH ( Table 17.2 ). AIS is defined as a localized, small (no more than 3 cm) adenocarcinoma consisting of neoplastic pneumocytes growing along preexisting alveolar structures (lepidic growth), lacking stromal, vascular, or pleural invasion ( Fig. 17.1B ). No papillary or micropapillary patterns should be present, and intra-alveolar tumor cells are absent. AIS is typically nonmucinous, consisting of type II pneumocytes and/or Clara cells, but rare cases of mucinous AIS do occur. The concept of AIS was proposed with the intention of defining lesions that should correlate with a 100% disease-free survival if completely resected. This proposal was supported by retrospective observational studies in tumors measuring either 2 cm or less or 3 cm or less. In the setting of multiple tumors, the criteria for AIS as well as MIA should be applied only if the other tumors are regarded as synchronous primaries rather than intrapulmonary metastases.
Minimally Invasive Carcinoma
MIA is defined as a small (no more than 3 cm), solitary adenocarcinoma with a predominantly lepidic pattern and invasion of no more than 5 mm in greatest dimension ( Fig. 17.1C–D ). MIA is usually nonmucinous, but it may rarely be mucinous. Measurement of the invasive component of MIA should include the following: (1) histologic subtypes other than a lepidic pattern (i.e., acinar, papillary, micropapillary, and/or solid) or (2) tumor cells infiltrating myofibroblastic stroma. MIA should not be diagnosed if the tumor invades lymphatics, blood vessels, or pleura or contains tumor necrosis. More details about measuring invasive size are explained elsewhere. The concept of MIA was introduced to define a population of patients who should have a 100% or near 100% 5-year disease-free survival rate if the lesion is completely resected. Although less evidence was found to support the concept of MIA compared with AIS, all published cases using these criteria have shown a 5-year disease-free survival rate of 100%.
The diagnosis of AIS or MIA requires that the tumor be completely sampled histologically (i.e., the patient has undergone a surgical resection). Both lesions should also have a discrete circumscribed border without miliary spread of small foci of tumor into adjacent lung parenchyma and/or with lobar consolidation. Review of CT scans may be helpful in evaluating pathologic specimens because the extent of ground-glass (usually lepidic) and solid (usually invasive) patterns can guide pathologists in assessing whether the lesion has been properly measured and/or sampled. For lesions suspected to be AIS or MIA larger than 3 cm, the term lepidic predominant adenocarcinoma is best applied, along with a comment that an invasive component cannot be excluded, because the data are insufficient to show that such patients will have 100% 5-year disease-free survival.
Invasive Adenocarcinoma
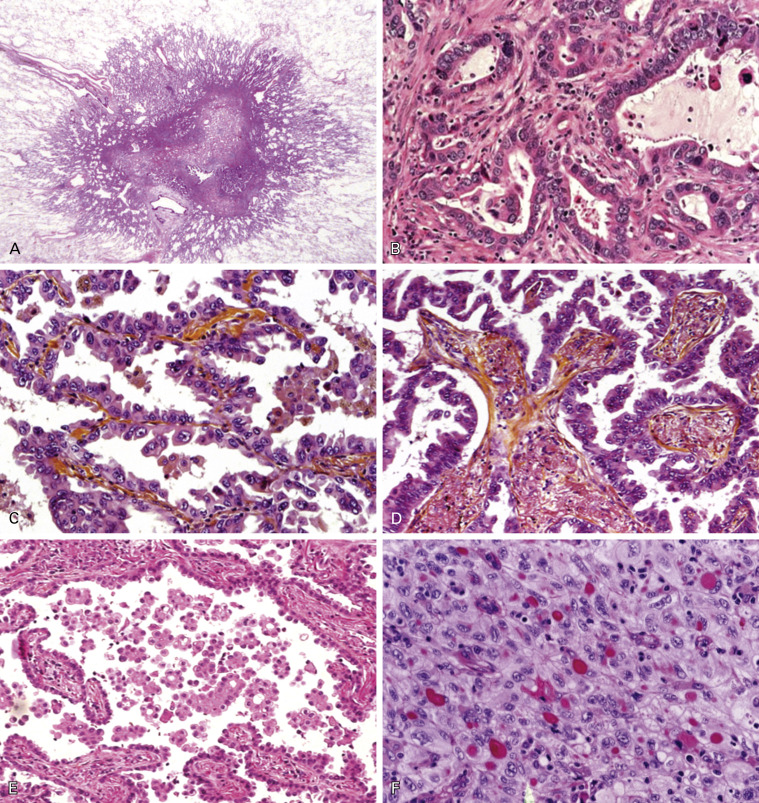
Because of the rarity of AIS and MIA, overtly invasive adenocarcinomas represent more than 70% to 90% of surgically resected lung adenocarcinomas. These tumors typically consist of a complex, heterogeneous mixture of histologic patterns, thus explaining the former category of adenocarcinoma, mixed subtype ( Fig. 17.2 ). The major subtypes of invasive adenocarcinoma are now classified according to the predominant component, after performing comprehensive histologic subtyping ( Fig. 17.2B–F , and Table 17.2 ). Comprehensive histologic subtyping is performed by making a semiquantitative estimation of each of the patterns in 5% increments. It is useful to record in diagnostic reports each adenocarcinoma subtype that is present with the respective percentages. This approach may also provide a basis for architectural grading of lung adenocarcinomas. Since the 2011 classification was initially published, a growing number of studies of resected lung adenocarcinomas have demonstrated its utility in identifying significant prognostic subsets and molecular correlations according to the predominant patterns.
Lepidic Predominant Invasive Adenocarcinoma
This subtype consists of a proliferation of bland type II or Clara cells growing along the surface of alveolar walls, similar to the morphology defined in the earlier section on AIS and MIA ( Fig. 17.2C ). Invasive adenocarcinoma is present in at least one focus measuring more than 5 mm in greatest dimension. Invasion is defined as follows: (1) histologic subtypes other than a lepidic pattern (i.e., acinar, papillary, micropapillary, and/or solid); (2) myofibroblastic stroma associated with invasive tumor cells; (3) vascular or pleural invasion; and (4) spread through alveolar spaces (STAS). Lepid predominant adenocarcinoma is diagnosed rather than MIA if the cancer invades lymphatics, blood vessels, or pleura or contains tumor necrosis. Several recent studies of early-stage adenocarcinomas published since 2011 have demonstrated that lepidic predominant tumors have a favorable prognosis, with 5-year disease-free survival rates of 86% to 90%. The term adenocarcinoma with lepidic pattern corresponds to some cases previously referred to as adenocarcinoma with BAC features. The term lepidic predominant adenocarcinoma should not be used in the context of invasive mucinous adenocarcinoma with predominant lepidic growth; these tumors should be classified as mucinous adenocarcinomas.
Acinar Predominant Invasive Adenocarcinoma
This subtype shows a major component of glands that are round to oval with a central luminal space surrounded by tumor cells ( Fig. 17.2B ). The neoplastic cells and/or glandular spaces may contain mucin. Cribriform structures can be found in the acinar pattern, are considered to be high grade, and are associated with poor prognosis. Tumor cells may form aggregates of polarized cell without clear lumen, which is still recognized as an acinar pattern.
Papillary Predominant Adenocarcinoma
This subtype shows a major component of a growth of glandular cells along central fibrovascular cores ( Fig. 17.2D ). If a tumor has lepidic growth, but the alveolar spaces are filled with papillary or micropapillary structures, the tumor is classified as papillary or micropapillary adenocarcinoma, respectively.
Micropapillary Predominant Adenocarcinoma
This subtype has tumor cells growing in papillary tufts or florets that lack fibrovascular cores ( Fig. 17.2E ). These may appear detached and/or connected to the alveolar walls. The tumor cells are usually small and cuboidal with minimal nuclear atypia. STAS is a newly suggested pattern of invasion, often seen with the micropapillary pattern; it can occur with micropapillary clusters, solid nests, or single cells. STAS probably contributes to the high recurrence rate for patients with small stage I adenocarcinomas who undergo limited surgical resections and the poor prognosis observed by others.
Solid Predominant Invasive Adenocarcinoma
The solid subtype with mucin production consists of a major component of polygonal tumor cells forming sheets but without any clear acinar, papillary, micropapillary, or lepidic growth ( Fig. 17.2F ). If the tumor is 100% solid, intracellular mucin should be present in at least five tumor cells in each of two high-power fields, confirmed with histochemical stains for mucin. Tumors formerly classified as large cell carcinomas that have immunohistochemical expression of TTF-1 and/or napsin A, even if mucin is not identified, are now classified as solid adenocarcinomas. Solid adenocarcinomas must be distinguished from nonkeratinizing squamous cell carcinomas and large cell carcinomas, both of which may show rare cells with intracellular mucin. Neuroendocrine markers, such as neural cell adhesion molecule (NCAM)/CD56, dense core granule associated protein chromogranin A, and synaptic vesicle protein synaptophysin should be performed only if neuroendocrine morphology is present, to allow the diagnosis of large cell neuroendocrine carcinoma (LCNEC).
Variants of Invasive Adenocarcinoma
Four variants of clinically relevant invasive lung adenocarcinomas are recognized: (1) invasive mucinous adenocarcinoma, with tumor cells that have a goblet or columnar cell morphology with abundant intracytoplasmic mucin ( Fig. 17.3A ); (2) colloid adenocarcinoma, with abundant mucin pools filling alveolar spaces; (3) fetal adenocarcinoma, resembling fetal lung; and, (4) enteric adenocarcinoma, an adenocarcinoma of the lung resembling enteric adenocarcinoma.
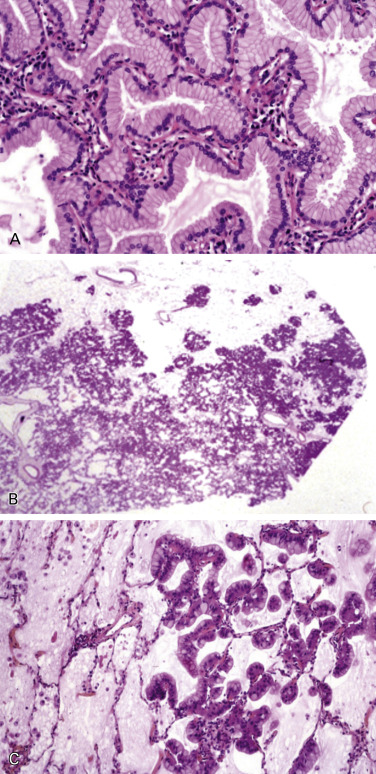
Invasive Mucinous Adenocarcinoma
Multiple studies have shown major differences in clinical, radiologic, pathologic, and genetic features between invasive mucinous adenocarcinomas and the tumors formerly classified as nonmucinous BAC. Invasive mucinous adenocarcinomas have tumor cells with a goblet or columnar cell morphology with abundant intracytoplasmic mucin and aligned, basally located nucleoli ( Fig. 17.3A ). This pathognomonic cellular feature can be recognized in small lung samples. Similar to nonmucinous tumors, invasive mucinous adenocarcinomas may show the same heterogeneous mixture of lepidic, acinar, papillary, micropapillary, and solid growth, which will not be described in detail and quantified for this specific subtype. Although invasive mucinous adenocarcinomas frequently show lepidic predominant growth, extensive sampling usually shows invasive foci. Therefore, reports on biopsy specimens should include a remark such as mucinous adenocarcinoma with lepidic pattern. However, if a resection specimen of the mucinous tumor fulfills the diagnostic criteria of AIS or MIA, the tumor should be diagnosed as mucinous AIS or MIA, respectively, although such tumors are extremely rare. In some cases, mucinous adenocarcinoma appears in CT scans and pathology specimens with a pseudo-pneumonia growth pattern ( Fig. 17.3B–C ).
Colloid Adenocarcinoma
This subtype shows abundant extracellular mucin in pools, which distend the alveolar spaces and destroy their walls, showing an overtly invasive growth pattern into the alveolar spaces. Mucin deposits enlarge and dissect the lung parenchyma, creating pools of mucin-rich matrix, while tumor elements consist of foci of tall columnar cells with goblet-like features growing in a lepidic fashion. Tumor glands often float into the mucoid material, becoming poorly recognizable and then requiring extensive tumor sampling.
Fetal Adenocarcinoma
This subtype consists of complex glandular structures composed of glycogen-rich, nonciliated cells resembling a developing epithelium in the pseudoglandular phase of the fetal lung, with low nuclear atypia and morule formation.
Primary Pulmonary Adenocarcinoma With Enteric Differentiation
This term is used to indicate a primary lung cancer resembling colorectal adenocarcinoma metastatic to the lung. Its histologic characteristics include eosinophilic tall columnar cells with brush border, vesicular nuclei, central geographic or dotted necrosis, occasional central scar and pleural indentation, and papillo–tubular (or gland-like) structure. The histologic resemblance to colorectal cancer is a hallmark of this tumor. Although some tumors have enteric differentiation with positive immunohistochemical expression of CDX-2 (which encodes for an intestine-specific transcription factor) and cytokeratin (CK) 20, and negative expression of CK7, others have only enteric morphology.
Immunohistochemistry of Adenocarcinomas
The immunohistochemical expression of lung adenocarcinomas varies somewhat based on the subtype and degree of differentiation. TTF-1 and napsin A are nearly specific markers for lung adenocarcinoma, except that thyroid cancer expresses TTF-1 and renal cell carcinoma expresses napsin A. Approximately 75% of invasive adenocarcinomas are positive for TTF-1 ( Fig. 17.4 ), and none of the squamous cell carcinomas express TTF-1. Among the adenocarcinoma subtypes, most lepidic and papillary predominant tumors are positive for TTF-1, as are the lepidic components of AIS and MIA, whereas positive frequency is lower in cases of solid predominant cancer. The sensitivity of napsin A is comparable to that of TTF-1, but its specificity is far lower. CK7 is another marker for lung adenocarcinoma, and its sensitivity, but not its specificity, is higher than that of TTF-1 and napsin A. Furthermore, tumor protein p63, which has been used as a marker for a squamous cell carcinoma, is also positive in some lung adenocarcinoma (up to 38%), and a portion of these adenocarcinomas are positive for anaplastic lymphoma kinase ( ALK ) gene rearrangement. In contrast, protein p40, an isoform of p63, 50 is never positive in adenocarcinomas except for those contained in adenosquamous carcinomas. Some adenocarcinomas may show a squamous appearance. In these cases, phenotyping with a limited panel of immunomarkers (including p40, and TTF-1) and mucin stain is necessary.
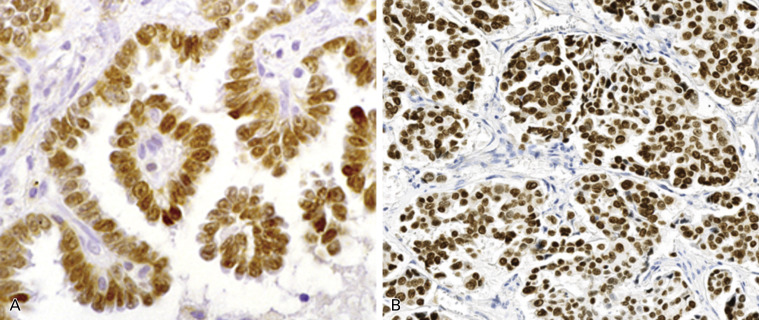
Histologic and Molecular Correlations
Despite the discovery of multiple molecular abnormalities in lung adenocarcinoma, no significant, specific histologic and molecular correlations have been found. A number of driver gene alterations are now known to exist in lung adenocarcinomas including mutations of EGFR, KRAS, BRAF, and ERBB2/HER2, and rearrangements of ALK, RET, ROS1, NTRK1, and NRG1. Among these mutations, EGFR and ALK are clinically relevant because molecular targeted drugs can be used in patients whose tumors have these molecular abnormalities. Adenocarcinomas with BRAF, HER2, ROS1, and NTRK1 abnormalities share clinicopathologic features with the EGFR-mutant and ALK-rearranged tumors in terms of involvement that is nearly specific to adenocarcinoma in lung cancer, particularly frequent in TTF-1–positive expression, and preferentially present in never-smokers and in women. The frequent finding of KRAS mutation and consistent lack of EGFR mutation in invasive mucinous adenocarcinoma is the strongest histologic and molecular correlation found in lung cancer. Most histologic subtypes of adenocarcinoma harbor EGFR and KRAS mutations, as well as ALK rearrangement. EGFR mutations are encountered most frequently in nonmucinous adenocarcinomas with a lepidic or papillary predominant pattern, whereas KRAS mutations tend to be found in solid predominant adenocarcinomas. ALK rearrangement has mostly been associated with an acinar pattern including a cribriform morphology and with signet-ring cell features, particularly in those tumors with TTF-1 and p63 coexpression.
Impact of the New Classification on Tumor, Node, Metastasis Staging
The 2011 IASLC/ATS/ERS classification of adenocarcinoma can affect tumor, node, metastasis (TNM) staging in several ways. First, it may help in comparing histologic characteristics of multiple lung adenocarcinomas to determine whether they are intrapulmonary metastases or separate primaries. Using comprehensive histologic subtyping along with other histologic characteristics has been shown to correlate well with molecular analyses and clinical behavior. Second, it may be more meaningful clinically to measure tumor size in lung adenocarcinomas that have a lepidic component by using the invasive size rather than total size to determine the final size of the tumor for TNM staging. It is possible that in the next edition of the TNM, AIS may be regarded as tumor carcinoma in situ (Tis) and MIA may be regarded as tumor microinvasive (Tmi).
Small Biopsy and Cytology Samples
In the past, NSCLCs were lumped together without attention to more specific histologic typing (e.g., adenocarcinoma, squamous cell carcinoma, etc.). One of the major new proposals in the IASLC/ATS/ERS classification was the development of standardized criteria and terminology for the pathologic diagnosis of lung cancer in small biopsy and cytology specimens ( Table 17.3 ). In addition to the criteria and terminology, two paradigm shifts have emerged for pathologists in terms of tumor classification and management of specimens. The first is the need to perform immunohistochemistry to further classify tumors formerly diagnosed as NSCLC not otherwise specified (NOS). Because the distinction between histologic types of lung cancer, particularly adenocarcinoma and squamous cell carcinoma, is so important, the new classification recommends that pathologists use special stains to try to further subtype carcinomas that are difficult to classify by light microscopic evaluation of histologic sections alone.
| 2004 WHO Classification Including Updated 2011 IASLC/ATS/ERS Terminology | Morphology/Stains | IASLC/ATS/ERS Terminology for Small Biopsy and Cytology Specimens |
|---|---|---|
| Adenocarcinoma Mixed subtype Acinar Papillary Solid | Morphologic adenocarcinoma patterns clearly present | Adenocarcinoma (describe identifiable patterns) |
| Lepidic (nonmucinous) | Adenocarcinoma with lepidic pattern (if pure, add note: an invasive component cannot be excluded) | |
| Lepidic (mucinous) | Invasive mucinous adenocarcinoma (describe patterns present; use term mucinous adenocarcinoma with lepidic pattern if pure lepidic pattern) | |
| No 2004 WHO counterpart; most will be solid adenocarcinomas | Morphologic adenocarcinoma patterns not present (supported by special stains, i.e., positive TTF-1) | NSCLC, favor adenocarcinoma |
| Squamous cell carcinoma | Morphologic squamous cell patterns clearly present | Squamous cell carcinoma |
| No 2004 WHO counterpart | Morphologic squamous cell patterns not present (supported by special stains, i.e., positive p40) | NSCLC, favor squamous cell carcinoma |
| Large cell carcinoma | No clear adenocarcinoma, squamous, or neuroendocrine morphology or staining pattern | NSCLC-NOS b |
a From Travis et al, with permission: Travis WD, Brambilla E, Noguchi M, et al. Diagnosis of lung cancer in small biopsies and cytology: implications of the 2011 International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification. Arch Pathol Lab Med. 2013;137(5):668–684.
b IASLC/ATS/ERS , International Association for the Study of Lung Cancer, American Thoracic Society, and European Respiratory Society; NSCLC-NOS , nonsmall cell lung cancer, not otherwise specified. Pattern can be seen not only in large cell carcinomas, but also when the solid, poorly differentiated component of adenocarcinomas or squamous cell carcinomas does not express immunohistochemical markers or mucin; TTF-1 , thyroid transcription factor-1; WHO , World Health Organization.
For tumors with classic morphologic features the diagnostic terms adenocarcinomas and squamous cell carcinomas can be used ( Table 17.3 ). The morphologic features of these tumors are described in detail elsewhere. If an NSCLC does not show clear glandular or squamous morphology in a small biopsy or cytology specimen, it is classified as NSCLC-NOS. Tumors with this morphologic appearance should be studied with a limited special stain workup in an attempt to classify them further. It is recommended to use a single adenocarcinoma marker (e.g., TTF-1), a single squamous marker (e.g., p40), and/or mucin stain. Tumors that are positive for an adenocarcinoma marker or mucin are classified as NSCLC, favor adenocarcinoma. Tumors that are positive for a squamous cell carcinoma immunohistochemical marker with negative adenocarcinoma markers are classified as NSCLC, favor squamous cell carcinoma. Cytology is a powerful diagnostic tool that can accurately subtype NSCLC in most cases, and immunohistochemistry is readily available when cell blocks are prepared for the cytology samples.
Squamous Cell Carcinoma
Squamous cell carcinoma is thought to arise from the airway epithelium and is characterized histologically by keratinization and/or intercellular bridges. If these morphologic hallmarks are absent, a diagnosis can be established only by positive immunohistochemical staining for markers such as p40 and CK5/6.
Squamous cell carcinoma usually arises in a main or lobar bronchus, but more peripheral locations are not uncommon. The centrally located hilar-type occasionally shows intraepithelial spread that may extend to the cut end of the resected bronchus; therefore, frozen sections of the mucosa at the resected end must be examined during surgical procedures. In comparison with adenocarcinoma, many peripherally located squamous cell carcinomas are only locally invasive, and pleural carcinomatosis is rare. The spread of squamous cell carcinoma is similar to that of other NSCLCs. Staging of squamous cell carcinoma, as for other malignant epithelial tumors of the lung, is performed according to the TNM system. Uncommonly, squamous cell carcinoma manifests as a superficial spreading tumor on the bronchial mucosa. Squamous cell carcinoma tends to be locally aggressive, involving adjacent structures through direct invasion.
Preinvasive Lesions
The histopathologic sequence of preinvasive lesions of squamous cell carcinoma has been identified and is characterized for different degrees of squamous dysplasia and carcinoma in situ ( Fig. 17.5 ). The criteria for squamous dysplasia and carcinoma in situ are similar to those for lesions occurring in the uterine cervix and oral cavity. However, the histologic appearance of these lesions is not uniform, because bronchial dysplasia originates from pseudostratified columnar epithelium and is not deeply associated with human papilloma virus (HPV) infection.
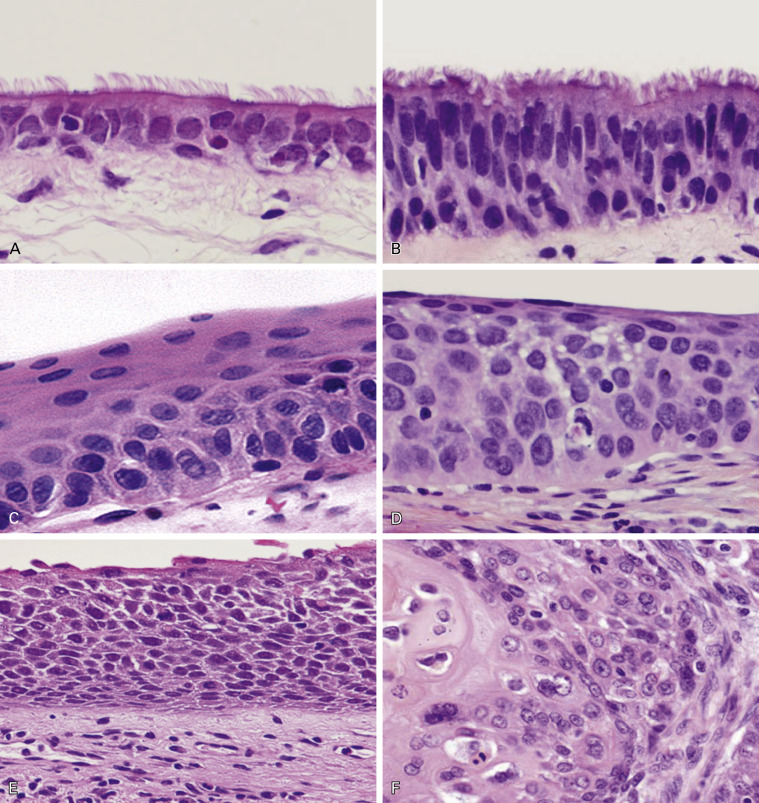
In a dysplastic lesion, the ciliated respiratory epithelium is replaced by thick, stratified squamous epithelium with moderate nuclear atypia, but still retaining the capacity for squamous differentiation. Angiogenic squamous dysplasia is a specific phenotype of dysplasia characterized by proliferation of small blood vessels in the submucosa; this subtype shows high proliferative activity and is associated with high-risk smokers. Carcinoma in situ is composed of stratified epithelium (more than 10 cells thick) with an increased nuclear-to-cytoplasmic ratio and severe nuclear atypia, but it does not show invasive growth.
Early Invasive Squamous Cell Carcinoma
Centrally located (hilar-type) early invasive squamous cell carcinoma is defined as a tumor that arises in areas up to the subsegmental bronchus, is confined to the bronchial wall, and shows no lymph node metastasis. Early invasive squamous cell carcinoma is associated with a 5-year survival rate of more than 90% and can be divided into the following subtypes: (1) polypoid type, frequently arising at the bronchial spar; (2) nodular type, arising at any bronchus and having a tendency to form a localized tumor showing vertical invasive growth; and, (3) superficially infiltrating type, showing in situ and microinvasive growths, often involving a wide area but exhibiting little tendency to produce bronchial stenosis and obstruction.
In comparison with the hilar-type, little is known about the peripheral type of squamous cell carcinoma. A unique subtype of squamous cell carcinoma with alveolar space filling (ASF) has been proposed. Peripheral-type squamous cell carcinoma can be divided into two distinctive subtypes, the ASF-type and the expanding or destructive-type, based on the condition of the elastic fiber framework. The ASF-type shows growth that fills the alveolar space without destruction of the existing alveolar structure or elastic fiber network ( Fig. 17.6A ). It has been suggested that ASF growth represents an in situ lesion with an extremely favorable prognosis.
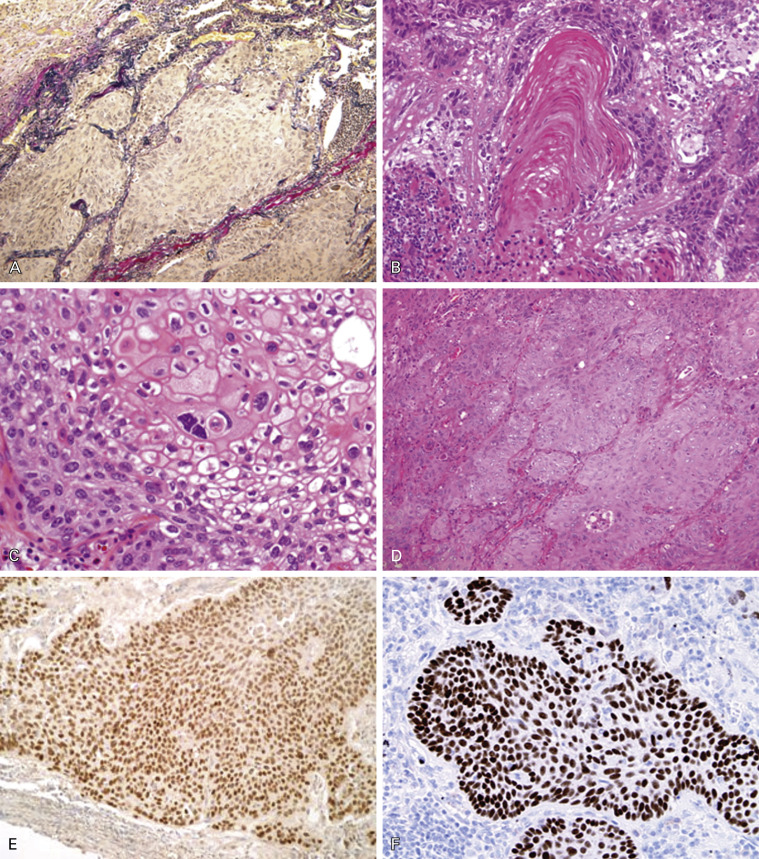
Invasive Squamous Cell Carcinoma
Invasive squamous cell carcinoma arises in both the major (main to segmental) bronchus and the peripheral parenchyma. The former type shows both endobronchial and invasive growth into the peribronchial soft tissue, lung parenchyma, and nearby lymph nodes. Squamous cell carcinoma growing in the endobronchial region sometimes blocks the bronchus, resulting in secondary changes to the distal lung, such as collapse, lipid pneumonia, and bronchopneumonia. On the other hand, squamous cell carcinoma arising in the peripheral region shows two different tumor growth types: the ASF-type and the compression-type. In comparison with the hilar-type, the peripheral squamous cell carcinoma frequently possesses mucin-containing cells and shows glandular cell charactertistics. Therefore, many tumors diagnosed as peripheral-type squamous cell carcinomas are actually adenosquamous cell carcinomas in the strict sense.
Invasive squamous cell carcinomas are grouped in three major histologic types, including the common types keratinizing and nonkeratinizing, and the basaloid type.
Keratinizing and Nonkeratinizing Squamous Cell Carcinoma
Most invasive squamous cell carcinomas are moderately or poorly differentiated. The well-differentiated carcinoma is not common in comparison with squamous cell carcinoma of stratified squamous epithelial origin, such as that of the oral cavity, pharynx, and esophagus. Squamous cell carcinoma arising from a major bronchus frequently shows an intraepithelial, in situ type of extension along the bronchus. Histologically, invasive squamous cell carcinoma shows geographic nests composed of polygonal cells with intercellular bridges and keratinization ( Fig. 17.6B–C ). As stated, the common type of squamous cell carcinoma is divided into two classes: keratinizing and nonkeratinizing. The keratinizing type is well to moderately differentiated and easy to diagnose as squamous cell carcinoma, whereas the nonkeratinizing type is sometimes difficult to diagnose if it does not contain intercellular bridges ( Fig. 17.6D ).
Keratinization and intercellular bridges are the hallmarks for the differential diagnosis of squamous cell carcinoma from other NSCLCs. However, if a tumor is poorly differentiated and differentiation is unclear, immunohistochemical analysis using a limited panel of markers and mucin staining is necessary. The most important diagnostic immunohistochemical marker of squamous cell carcinoma is p40 ( Fig. 17.6F ), which is more specific than p63 ( Fig. 17.6E ). These markers are positive in the tumor cell nuclei and are fundamental markers for basal cells of the bronchial mucosa. CK5/6 is a less reliable marker of squamous cell carcinoma. TTF-1, which is a very specific marker for adenocarcinoma, should be negative. If the immunostaining profile is the only evidence for a diagnosis of squamous cell carcinoma, such cases should be diagnosed as tumor with squamous cell carcinoma nonkeratinizing type. It is sometimes difficult to differentiate pulmonary squamous cell carcinoma from metastatic squamous cell carcinoma of other sites, such as the head and neck, esophagus, or cervix. Genotypic fingerprinting for TP53 mutation, loss of heterozygosity, or HPV genotyping has been reported to be useful for the differential diagnosis. Despite the discovery of multiple molecular abnormalities in squamous cell carcinoma, no significant, specific histologic and molecular correlations have been found in this tumor type.
Immunohistochemistry of Squamous Cell Carcinoma
Squamous cell carcinoma has traditionally been defined as a tumor that shows keratinization, pearl formation, and/or intercellular bridges. Immunohistochemistry is not needed in these tumors. As intercellular bridging may be scarce in nonkeratinizing squamous cell carcinoma, immunohistochemistry is needed to distinguish theses tumors from large cell carcinoma with a null immunophenotype in surgical resections, and NSCLCs with adenocarcinoma or NSCLC-NOS phenotype on small biopsies. For such tumors, diffuse positive staining with p40, p63, and/or CK5 or CK5/6 confirms their squamous phenotype and classification as a nonkeratinizing squamous cell carcinoma. Both TTF-1 and mucin stains should be negative or only focally positive (for TTF-1, less than 10% of cells with faint staining).
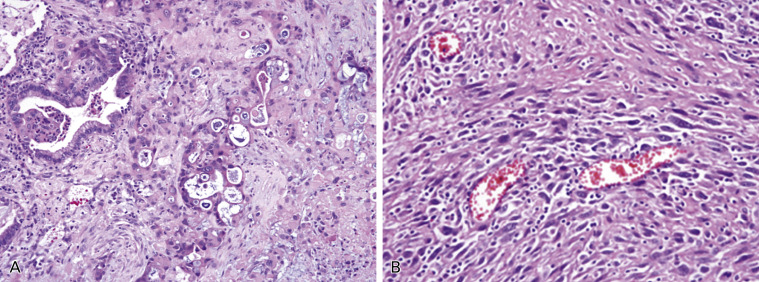
Basaloid Carcinoma
Basaloid carcinoma is a variant of squamous cell carcinoma ( Fig. 17.7 ). This is a poorly differentiated tumor that displays lobular architecture and peripheral palisading of nuclei at the edge of tumor nests and lacks squamous differentiation. The tumor cells are relatively small with scanty cytoplasm and absent or focal nucleoli. The mitotic rate is high (15–50 per 2 mm 2 ) and the proliferation index is high as assessed by Ki-67 (approximately 50–80%). In the 2004 WHO classification, this tumor was classified as the basaloid variant of large cell carcinoma; however, as it is usually positive for p40 immunohistochemical expression, recently it has been reclassified as a variant of squamous cell carcinoma ( Fig. 17.7B ). Tumors with keratinizing or nonkeratinizing squamous cell carcinoma features that have more than 50% basaloid component are classified as basaloid carcinoma. These changes were introduced to the new 2015 WHO classification. Tumor spread and staging of basaloid carcinoma are similar to those of other squamous cell carcinomas of the lung.
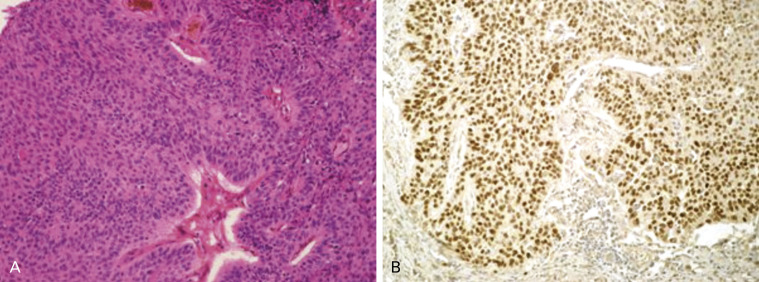
Immunohistochemistry of Basaloid Carcinoma
Basaloid carcinoma is a specific subtype of nonkeratinizing squamous cell carcinoma that requires differential diagnosis with SCLC and LCNEC, with which it might be confused in the case of crushed artifact due to small cell size or the presence of rosettes and palisading with centrolobular necrosis. Basaloid carcinoma consistently shows diffuse and strong expression of p63 and its isoform p40 ( Fig. 17.7B ). CK5/6 and the cytokeratins included in the antibody 34βE12 (CKs 1, 5, 10, and 14) are also expressed in all cases, sometimes in a less diffuse fashion. TTF-1 is never expressed. Neuroendocrine markers (NCAM/CD56, chromogranin A, and synaptophysin) are usually negative.
Adenosquamous Carcinoma
This tumor is characterized by the presence of both squamous cell and adenocarcinoma differentiations. A carcinoma showing histologic characteristics of each component in at least 10% of the tumor should be categorized as adenosquamous carcinoma ( Fig. 17.8A ). However, a situation where less than 10% of each histologic differentiation is present should be reported because recent molecular analyses have suggested that tumors with mixed features can reflect the genetic status of either component regardless of their proportion in the tumor. The frequency of adenosquamous carcinoma is estimated at 0.4% to 4% of lung cancers. Typically, adenosquamous carcinomas are located in the peripheral pulmonary parenchyma, but they have also been reported to arise centrally. The clinical characteristics are similar to those of other NSCLCs. The outcome of adenosquamous carcinoma is significantly poorer than that of adenocarcinoma and squamous cell carcinoma, particularly in stages I and II. The amount of each component does not affect the survival rate.