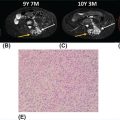
31524 Chordoma Chordomas are rare bone tumors accounting for ~4% of all bony malignancies. Clinically, these tumors typically grow slowly, but act in a locally invasive manner and usually occur along the axial and para-axial bones. Common locations of chordoma are the sacro-coccygeal spine, mobile spine, and skull base. The clinical management of chordomas requires a multidisciplinary approach to optimize the diagnosis and treatment plan. Expert pathologic consultation is required for this rare diagnosis. Curative surgery remains the standard treatment of chordomas. In patients with technically inoperable disease or in patients who decline resection, high-dose radiation is an option but may have a higher rate of local progression than the combination of surgery and radiation. Targeted therapy for locally advanced or metastatic disease is effective for tumor stabilization but has a low response rate using standard response evaluation criteria in solid tumors. This chapter discusses clinical presentation, radiographic findings, diagnostic approach, pathology, treatment, and management of disease recurrence for chordomas. chordomas, clinical studies, immunotherapy, radiation therapy, targeted therapy Chordoma, Clinical Study, Immunotherapy, Molecular Targeted Therapy, Radiotherapy INTRODUCTION Chordomas are rare bone tumors accounting for ~4% of all bony malignancies. The incidence of chordoma is approximated to be just under one case per million people and there are approximately 300 cases diagnosed per year in the United States.1–3 Chordomas are more common in men than women and typically affect people in the fourth decade of life. The 5-year overall survival (OS) rate is reported to range from 50% to 67%, depending on the stage of the disease and the presence of relapsed or metastatic disease.3,4 There are various hypotheses about the pathogenesis of chordomas. One prevailing theory is that chordomas derive from the remnants of the notochord, a midline embryonal structure that plays a role in neuronal differentiation, which usually regresses during fetal development.5–7 Currently, the molecular drivers of chordoma are still under investigation. Brachyury, vascular endothelial growth factor receptor (VEGFR), and c-KIT are putative oncogenic drivers.8,9 Clinically, these tumors typically grow slowly, but act in a locally invasive manner and usually occur along the axial and para-axial bones. Common locations of chordoma are the sacro-coccygeal spine, mobile spine, and skull base. Common clinical presentations, such as pain, mass effect, or cranial neuropathy vary based on the location and degree of tumor extension.4,10–12 The clinical management of chordomas requires a multidisciplinary approach to optimize the diagnosis and treatment plan. Expert pathologic consultation is required for this rare diagnosis. Curative surgery remains the standard treatment of chordomas. En bloc resection in conjunction with radiation is considered to be the most effective approach for localized disease.13–17 In patients with technically inoperable disease or in patients who decline resection, high-dose radiation is an option but may have a higher rate of local progression than the combination of surgery and radiation.17–20 Distant metastasis has been reported in 10% to 40% of patients and systemic chemotherapy has shown limited efficacy, but these have seen some promising results with the use of targeted therapy.21–25 PATHOGENESIS AND MOLECULAR PATHWAY The pathogenesis of chordomas remains unclear. Various hypotheses have been proposed and the prevailing notion is that these tumors develop from notochordal remnants. The notochord is formed on day 17 of embryogenesis and migrates cranially between the ectoderm and endoderm. During fetal development, the notochord is surrounded by adjacent neuro-ectodermal tissue and is replaced by cartilage cells. Its regression remnant becomes the nucleus pulposus of the intervertebral discs. Currently, several aberrant genetic changes have been proposed to be the oncogenic drivers of chordoma. Chromosomal Abnormalities Theoretically, the well-known gene called “T-gene” or brachyury was considered to play a major role of both familial and sporadic chordoma development. Duplications of the brachyury gene on chromosome7, polysomy-7, or chromosome7 translocations all can cause a gain-of-function of this proto-oncogene, which induces abnormal cell growth and inhibits apoptosis within notochordal remnant cells.26–29 Moreover, Patrick et al. have reported mutations in chromatin remodeling genes, such as CDKN2A and SETD2, in chordoma sequencing experiments.27,30 316Mutations in Chordomas A series of mutations have been found in chordomas, and it is unclear as yet how many are “driver” and how many are “passenger” mutations. Receptor tyrosine kinase overexpression has been detected in 77% to 99% cases.8,9,31–34 The platelet-derived growth factor receptor (PDGFR-α and –β), c-KIT, and VEGFR can be detected in endothelial cells and various types of normal or inflammatory tissues, but rarely is expressed in normal bone or chondrocytes. These mutations have been targeted in several small studies discussed later in the chapter. Several studies reported activating mutations in the PI3K/AKT/MTOR pathway in approximately 16% of chordomas. Mutations in this pathway are a potential predictive biomarker for PI3K/MTOR inhibitor treatment.27 The LYST (lysosomal trafficking regulator protein) regulates intracellular protein trafficking to the endosome. It was also discovered to be a novel gene that regulates the development of chordoma in approximately 10% of cases. Abnormal regulation of this gene has been shown to result in transcription variants. Epigenetic Change Epigenetic changes in chordoma cell lines and tissues have been documented. However, it is unclear at this time how to take advantage of these epigenetic changes. Abnormal DNA methylation of cytosine adjacent guanine (CpG) was identified in chordoma cell lines, resulting in the silencing of several tumor suppressor genes. Hyper/hypomethylation and acetylation of histones are also found in chordoma tissues. Finally, dysregulation of microRNA has been identified; putatively, abnormal expression of specific microRNAs could initiate chordoma and facilitate tumor progression and invasion.35–37 CLINICAL PRESENTATION The clinical presentation of chordomas is variable and depends on the tumor location and its extension into adjacent structures. Chordomas that arise in the mobile spine, sacrococcygeal area, and skull base all have different presentations. Typically, the clinical presentation is insidious, and most patients present with localized disease. The most common sites of metastatic disease include the lung, bone, or soft tissues, and metastasis most often occurs in patients with large primary tumors.18 Metastatic disease can also occur late. One caveat is that the dedifferentiated variant typically has a more aggressive behavior and often presents with metastatic disease. Spinal and Sacrococcygeal Tumor The most common site of chordoma is S4–S5 in the sacrum. An early stage sacral chordoma can be asymptomatic or present with nonspecific pain. Patients usually experience deep pain upon tumor progression and more extensive destruction of bone. With later presentation, neurologic deficits such as weakness, paresthesias, neuropathic pain, cauda equina syndrome or bowel/bladder sphincter dysfunction can be present. Constipation can occur from rectal compression. Signs and symptoms of tumor arising in the mobile spine are similar to those for other spinal tumors. When extraosseous extension occurs, usually from the vertebral body posteriorly into the spinal canal or neural foramina, back pain, leg weakness, sensory deficit, radicular pain, abnormal deep tendon reflexes, and bowel bladder dysfunction can be present. Patients with cervical spine tumor can present with Horner syndrome due to tumor compression of the cervical sympathetic chain.1,12,14,38 Intracranial Tumor Intracranial chordoma typically arises from the clivus. Headache and diplopia are the most common presenting symptoms in this situation. If the tumor extends superiorly, cavernous sinus syndrome can occur. Destructive skull base tumors can enlarge posteriorly leading to cranial neuropathies such as ophthalmopathy, trigeminal neuralgia, or facial numbness. Cerbrospinal fluid (CSF) rhinorrhea is less common. Epistaxis can occur if the tumor grows anteriorly into the nasopharynx.10,39,40 Intracranial hemorrhage, hypopituitarism, and pituitary apoplexy have also been reported.41 RADIOGRAPHIC FINDINGS CT and MRI are useful to assess the location and extension of tumor. The morphologic and contrast enhancement pattern can discriminate chordomas from the benign bone lesions, such as notochordal remnants, hematomas, bone cysts, and malignant bone tumors. 317CT Scan With Contrast CT is a highly sensitive and accurate imaging technique for bony lesions. Chordomas appear as a homogeneous mass isodense to adjacent soft tissue structures. In the contrast phase, the tumor develops heterogeneous enhancement. Calcification from bone sequestration or calcified tumor is a less common finding. These tumors are mostly central, are well circumscribed within expanded bone, and can have adjacent soft tissue extension. Bony destruction with marginal sclerosis is also common.10,42,43 MRI MRI is preferred to assess soft tissue extension and neurovascular bundle involvement over CT scan but is less sensitive to detect calcification and bony osteolysis. On MRI, chordomas show low to intermediate signal intensity in T1-weighted sequences and a vivid hypersignal intensity in T2-weighted images. With gadolinium, the tumor shows heterogeneous intratumoral enhancement with a pathognomonic “honeycomb” appearance corresponding to a low signal intensity in noncontrast T1-weighted images. Some specific radiographic findings in chordomas include expansion of bone, trabeculation, and rarefaction.10,43–45 FDG-PET/CT Little data is available on fluorodeoxyglucose (FDG)-PET in chordoma. Its role in tumor assessment, staging, and response evaluation is still to be defined.46 The accuracy of FDG-PET/CT for distinguishing between low-grade malignant bone tumor and benign tumors is still under investigation. Moreover, metastases smaller than 8 mm can cause false negative readings.47,48 DIAGNOSTIC APPROACH The current National Comprehensive Cancer Network (NCCN) recommendations are that chordomas and other bone malignancies should be evaluated by a multidisciplinary team at a high-volume center. Differentiating chordomas from common bone diseases is a first step in management. Tissue diagnosis is required before proceeding with curative intent. Core-needle biopsies are preferred over fine-needle aspirates as they allow the pathologist a better evaluation of the tissue architecture and help to discriminate from other bone tumors. Naturally, the biopsy technique depends on the tumor location but should be discussed in detail with the multidisciplinary team who will manage the patient to avoid inappropriate biopsy approach that may compromise planned surgery and/or radiation. Spinal tumors are readily accessible via CT-guidance or open biopsy; CT-guided core biopsy is preferred to avoid spillage of tumor. Intracranial tumors may require an endoscopic biopsy. PATHOLOGY Chordomas are classified into four different variants: conventional, chondroid, dedifferentiated, and poorly differentiated. Conventional variant is the most common subtype. Pathologic findings include lobules of cells arrange in cords and cohesive nests within a mucinous matrix. By immunohistochemistry, conventional chordomas typically express epithelial membrane antigen and epithelial markers. Most tumor cells are positive for S-100. Positive immunostaining for brachyury combined with cytokeratin provides a sensitivity and specificity of 98% to 100%.28,49 Chondroid variant is defined as the tumor containing an area of conventional chordoma next to a low-grade hyaline-type chondrosarcoma. Immunohistochemistry staining is often similar to the conventional subtype.50 Dedifferentiated variant is a very rare subtype reported in approximately 5% of cases.51 It contains a region of conventional chordoma with the areas of high-grade or poorly differentiated spindle cell sarcoma. Dedifferentiated chordomas can arise within de novo chordoma or change from the conventional type after recurrence or treatment. The tumor loses expression of brachyury and cytokeratin by immunohistochemistry. Dedifferentiated chordoma should be suspected in the cases of biopsy-proven conventional chordoma with aggressive clinical features.50 Poorly differentiated variant is a recently described subset of chordoma with an absence of INI1 (SNF5/SMARCB1) expression. It is prevalent in the pediatric population and has an aggressive clinical course.52 318STAGING Complete staging is recommended before definite treatment. CT imaging should be done to exclude distant metastasis to the common sites, such as the lung, soft tissue, and bone. MRI for screening of whole spine should be done because drop metastasis along the cerebrospinal axis can occur in skull base patients and other notochordal tumors, including synchronous chordoma, or benign notochordal tumors can be seen in some patients.53 Bone scans are considered a standard staging modality in malignant bone tumors. DIFFERENTIAL DIAGNOSIS The differential diagnosis for a chordoma includes benign bone lesions, malignant bone tumors, and bone metastases. MRI findings of chondrosarcomas can be similar to those for chordomas, but chondrosarcomas are more commonly located in the thoracic spine rather than the sacrococcygeal region. Ring or arc calcifications are characteristic of chondrosarcoma, but not as common in chordomas.54 Giant cell tumors of bone can be found in the sacrum but on MRI, they possess more heterogeneous signal intensity on T2-weighted imaging without marginal sclerosis or calcification.55 The differential diagnosis of skull base tumors includes chondrosarcoma of the skull base, but this is usually located in the paramidline region.56 Pituitary macroadenomas mostly shows isodensity on T1-weighted and T2-weighted MRI with area of necrosis or hemorrhage.57 Notochordal hamartomas of the skull base have similar imaging findings to chordomas, but the presence of stalk-like lesions at the prepontine region are more suggestive of hamartomas than chordomas.58 Bony metastases often involve multiple sites, show varying enhancement, and decreased signal intensity on T1-weighted MRI.59,60 Lymphomas are also usually multifocal, but primary bone lymphomas should be ruled out as well; lymphomas typically will exhibit high FDG uptake on PET scans. Plasmacytomas can occur as solitary bone or paramidline masses in the spine, sacrococcygeal region, or skull base with classic punched-out lesions with an associated extraosseous mass concurrent with or without clinical findings consistent with multiple myeloma.61 Histologically, chordomas are difficult to differentiate from benign notochordal tumors. The chondroid-type chordoma can be misdiagnosed as chondrosarcoma, which is negative for cytokeratins and brachyury by immunostaining.50 PROGNOSTIC FACTORS Properly validated prognostic factors for chordomas are limited by the rarity of this disease. The literature in this area mostly consists of retrospective analyses of clinical and molecular features of chordomas. Most studies in the literature are an amalgamation of cases from different primary locations, surgical approaches, rate of clear surgical margins, and varying adjuvant radiation doses. Several of these retrospective analyses revealed that tumor size, location of primary tumor, demographic data, and surgical margin are important prognostic factors. The analysis of the California Cancer Registry Database4 identified that in 409 chordoma patients, larger tumors were independently associated with a 2.5-fold increased risk of death. Hispanic race, high socioeconomic status, and patients undergoing surgery were reported to have a favorable outcome. The primary tumor location was not correlated with survival. Other studies have indicated that the location of primary tumor could impact survival as different locations place limits on the extent and type of surgeries that can be done. The 5-year OS in patients with intracranial primaries (67%–78%) was shorter than spinal primaries (90%–92%).4,62–65 The negative surgical margin (R0 resection) was an independent favorable prognostic factor compared to incomplete surgery, in line with most bone and soft tissue sarcoma series.62 Molecular prognostic markers have been analyzed to correlate the molecular profiles with recurrence of disease and survival. The expression of brachyury and increased copy number gain of 1q or 2p of T-gene were associated with significant shorter disease-free survival (DFS) and OS.66,67 MGMT promoter methylation was found to have a higher recurrent rate of clival chordoma compare to unmethylated tumor.68 The overexpression of p53 or CDK4 significantly correlated with poor OS.67 Homozygous 9p21 deletions and 1p36 deletions were found to be independent prognostic factors in clival chordoma.69 319TREATMENT The foundation of treatment for a chordoma is radical resection. The approach to each patient is individualized, but the goal should be to achieve negative margins with minimal surgical morbidity. However, this needs to be balanced with the fact that chordomas are usually deeply located and are locally invasive, so margin negativity need not be achieved if significant morbidity would result. Adjuvant radiation is also included in the NCCN guidelines. Radiation alone without surgery is associated with an inferior outcome compared to some surgical series, although this option may be chosen by some patients with upper sacral and cervical chordomas who are neurologically intact and might choose a lower rate of local control and avoidance of potential acute surgical morbidity Some radiation alone series, in fact, show better outcome than the frequently used paradigm of surgery and postoperative radiation, although the use of preoperative radiation as a component of high-dose proton-based radiation appears to be better than either definitive radiation or surgery with postoperative radiation.16,17,19,20,70 There is no current data for effective adjuvant systemic treatment. Systemic treatment should be considered for patients with recurrent or disseminated disease or in the setting of a clinical trial. SURGERY Spinal and Sacrococcygeal Tumor Several studies support a survival advantage with adequate surgery.13–15 A retrospective study of 501 patients demonstrated a significantly longer DFS after wide excision than marginal resection (75% vs. 33%).13 The surgical margin was regarded to be the most important prognostic factor and the majority of the patients with negative surgical margin remained disease-free long-term. On the contrary, all of the patients who underwent intralesional excision or inadequate dose radiation alone had recurrent disease within 2 years.14 More recent data have reported a 92% 5-year OS after surgery with significant longer DFS among patients with R0 resection.62 Recent data for definite radiation with high dose proton-based radiation therapy (RT) for unresected chordomas, primarily sacrococcygeal, reported 5-year local control of 85%.20 Similar efficacy has been reported for carbon ion radiotherapy for unresected sacral chordoma.71 An ongoing single-institution Phase II trial (ISAC/NCT01811394) being conducted in Heidelberg, Germany, randomizes patients with sacral chordoma (either unresected or following R2 resection) to scanned proton versus carbon ion RT, treating to 64 GyRBE in 16 fractions. Skull Base/Intracranial Tumor Intracranial chordomas are a technically challenging problem. Although radical resections provide long-term survival, surgical complications including neurologic morbidity and bleeding must be weighed against radicality of the surgical approach. Surgical complications that have been reported include cranial nerve palsy, swallowing dysfunction, intracranial hematoma, CSF leakage, hydrocephalus, and infection with an overall complication rate of approximately 10% to 25%. Only a small number of fatal complications have been reported in the literature.15,64,65,72,73 At the moment, surgical techniques for skull-based tumors vary according to tumor location, surgical expertise, and institutional preference. Radiation The local recurrence rate has been reported to be as high as 40% to 50% with radical surgery for chordomas.14,15,64,72 Effective RT may have a role to eradicate microscopic disease and diminish recurrence rate, although it is still not known how to best select patients for RT. Biologically, chordomas are relatively radioresistant tumors. Conventional doses of x-ray therapy are not felt to be effective and in general, high doses of radiation are used in the adjuvant setting. A systematic review reported the efficacy and safety of adjuvant RT in the patient with spinal and sacral chordoma.74 The study by Zabel-du Bois et al. demonstrated the patients receiving intensity-modulated photon-beam radiation (IMRT) dose over 60 Gy had a significant lower rate of local recurrence and improved OS compared to doses below 60 Gy.75 A prospective Phase II study by DeLaney et al. reported 5-year local control rate with combination adjuvant photon/proton radiotherapy was 94% at the median radiation dose of 76 cobalt Gy equivalent.76 Timing of adjuvant radiation may affect the local recurrence rate. One retrospective study found 5-year local control rate was 88% in early radiation group compared with 9% in the patients who received radiation for salvage or recurrence disease.77 High-dose single or hypofractionated RT has also been reported to be effective for this relatively radioresistant tumor. A study by Yamada et al. revealed the 2-year local control rate of 95% of adjuvant 320single fraction RT with median dose 2,400 cGy.78 However, delivery of high-dose photon beam can cause increased toxicity to surrounding tissues. Studies of proton beam therapy were conducted to allow a higher radiation dose to the target volume with minimized doses to surrounding structures. DeLaney et al. demonstrated that the local control rate among 36 patients with primary spine sarcomas, including chordoma, was 88% at 8 years with median radiation dose of 76.6 Gy. Recurrence occurred in only one of 23 patients with primary chordomas treated with adjuvant, neoadjuvant, or definitive high-dose proton/photon radiation.76 However, surgical hardware can potentially affect the accuracy of the proton dose to the target volume. Several studies reported a higher 5-year local recurrence rate in the patients who had titanium-based stabilization hardware.76,79 Heavy ion particle radiation may have higher biologic effectiveness because of higher linear energy transfer along the particle track, resulting in denser DNA damage, more double-stranded DNA breaks, and greater effectiveness against hypoxic cells. Studies with carbon ions report local control of 85% to 89% at 2 to 5 years.71,80 In patient with intracranial chordomas, the benefit of adjuvant radiation remains unclear. Retrospective studies by Choy et al. revealed that adjuvant RT improved 5-year local control from 20% to 62% in patients with subtotal resection of clival chordoma.81 Another study showed the 4-year local control rate of 56% with adjuvant photon/proton radiation at median dose of 67 Gy.82 The toxicities of RT depend on the location and kind of radiation treatment (that is, particle vs. photon). Acutely, these may include fatigue, mucositis, and diarrhea. Late bowel–bladder dysfunction related to cauda equina or sacral nerve injury and other kinds of neurotoxicity, while possible, are uncommon in the modern treatment era. DeLaney et al. reported no nerve injuries among 25 patients treated in the adjuvant radiation setting with median dose of 70.2 Gy and four nerve injuries (two grade 3 sacral neuropathies and two others [grade 2 in one and grade 3 in the other]) and erectile dysfunction among 25 patients with gross disease after surgery or biopsy, treated to 77.4 Gy, many of whom had upper sacral tumors, where resection would have resulted in 100% chance of nerve injury.76 Other potential late effects of radiation include sacral insufficiency fracture, rectal bleeding, pituitary dysfunction, and (rare) radiation associated secondary malignancy. FOLLOW-UP AND SURVEILLANCE The 2019 NCCN guidelines for surveillance after treatment of chordoma include history and physical examination every 6 months out to 5 years and then annually. Surveillance chest imaging is recommended every 6 months and may include an annual CT for 5 years and annually thereafter, but there may be some variations in practice among different institutions. Imaging of primary site includes x-ray studies or MRI as clinically indicated. MANAGEMENT OF DISEASE RECURRENCE Loco-Regional Treatment The most common site of recurrence is in the loco-regional area. According to the best practice for management of loco-regionally recurrent chordoma by the Global Consensus Group, salvage resection should aim to achieve gross total removal with negative margins. The success rate of re-resection varies among retrospective analyses ranging from 32% to 100%. The 5-year OS depends on the quality of surgery with or without radiation, but it is uniformly acknowledged to be less successful than that achieved at the time of initial treatment.70 Adjuvant radiation is recommended in patients without prior RT. In the patient with no prior RT, high-dose radiation with curative intent as an alternative to surgery or combined with maximal tumor resection or RT alone is indicated. No direct comparison data exist to determine the advantage of single versus combined treatment modality in this setting. A retrospective data report noted the impact of high-dose proton/photon beam RT on recurrent tumor; the 5-year DFS was 43% and 25% with surgery and nonsurgery, respectively.16 In patients with recurrence disease after primary adjuvant radiation, reirradiation can cause dose-limiting toxicity to adjacent organs. Reirradiation can be considered if the projected radiation dose to target volume is judged adequate for disease control with an acceptable risk toxicity. McDonald et al. reported proton reirradiation of 13 patients with an estimated 2-year rate of local control of 85%, although late toxicity included grade 3 bitemporal lobe radionecrosis in one patient that improved with hyperbaric oxygen, a grade 4 CSF leak with meningitis in one patient, and a grade 4 ischemic brainstem stroke (out of radiation field) in one patient, with subsequent neurologic recovery.83 If definitive 321radiation and/or surgery is judged not feasible, palliative radiation or palliative surgery followed by radiation for symptom alleviation is reasonable. Systemic Treatment For patients with locally advanced disease or distant metastasis who are not candidates for loco-regional treatment, systemic therapy is indicated, although the timing for systemic therapy must be carefully considered, because curative therapy is not currently available and patients, particularly with metastatic disease, are often asymptomatic and there is often an initially indolent rate of growth. Because growth is often indolent, clinicians and patients may have the luxury to delay treatment until patients have clearly measurable disease to assess the effectiveness of any therapy as well as the opportunity to investigate and participate in available clinical trials that can generally be accessed through the clinicaltrials.gov website. As noted below, “response” assessment in chordoma may be a challenge because of the often indolent rate of tumor growth, and “activity” may be manifested only in a change in tumor growth kinetics, including cessation of growth. The time from diagnosis to metastasis was reported in one series to range from 0.2 to 13 years.84 The median OS from diagnosis of metastasis to death was 22.1 months regardless of treatment modality.21 The rationale for the choice of systemic may be based on the molecular characteristics of chordomas. Conventional chordomas are generally insensitive to cytotoxic chemotherapy. However, a single Phase II study by Chugh et al. using 9-nitro-camptothecin in 15 advanced chordoma patients reported a median time to tumor progression of 9.9 weeks and objective response rate of 7%.85 A case series by Dhall et al. showed four patients with durable disease control for 9 to 13 years after subtotal resection of conventional clival chordoma followed by ifosfamide plus etoposide.86 Fleming et al. showed a complete response in a dedifferentiated chordoma patient with lung metastasis using a six-drug regimen of etoposide, cisplatin, vincristine, dacarbazine, cyclophosphamide without recurrence with 24 months of follow up.87 Several cases reports using multiple drugs including carboplatin/paclitaxel, liposomal doxorubicin/thalidomide, and VAC/IE (vincristine, doxorubicin, cyclophosphamide, followed by ifosfamide and etoposide) had a durable response (complete or partial response) up to 12 months.88–90 Discovery of oncogenic tyrosine kinase receptor pathways led to the development of clinical trials of targeted therapy. VEGFR, PDGFR, c-KIT, or MTOR pathways have been implicated in chordoma oncogenesis, which can be targeted by various TKIs. Imatinib, a multi-kinase inhibitor, has the most promising clinical activity for metastatic chordoma. A prospective Phase II study was conducted by Stacchiotti et al. using imatinib 400 mg twice daily in 56 patients with PDGFR/PDGR positive tumors. The objective response rate was only 2%, but the disease stabilization rate was 72%. The median PFS was 9.2 months and the median OS was 34.9 months.23 Another prospective study using dual blockade with imatinib plus everolimus was recently published and included 43 patients with progressive imatinib-pretreated advanced chordomas. The data showed a clinical benefit rate (patients responding or demonstrating absence of growth) of 88%. The median PFS was 14 months and the median OS was 47 months.91 This clinical data indicate that imatinib should be considered as a possible first-line systemic option for advanced/unresectable disease. In addition, epidermal growth factor receptor (EGFR) and HER-2 expression was found to be a predictive biomarker for the use of EGFR targeting TKIs. The efficacy of lapatinib was reported in a Phase II prospective trial of 18 imatinib-pretreated patients who had EGFR overexpression tumor. Lapatinib at a dose of 1,500 mg/day offered a disease control rate of 72%24 (Tables 24.1 and 24.2).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree