In the four decades since the introduction of MOPP (mechlorethamine, vincristine, procarbazine, and prednisone) by DeVita and coworkers,
1 significant advances have been made in enhancing efficacy and ameliorating the adverse effects of chemotherapy in Hodgkin lymphoma. Multidrug chemotherapy is now firmly established as the basis for curative treatment in patients with advanced-stage disease, and its role has been expanded to include its use in those with localized disease as well. In 2010, there were nearly 8,500 new cases of Hodgkin lymphoma diagnosed in the United States and approximately 1,300 deaths.
2 This translates into an annual age-standardized incidence rate of 2.8 per 100,000 and a death rate of approximately 0.4 per 100,000, down from 1.8 before the introduction of MOPP. It is increasingly clear, however, that many survivors of Hodgkin lymphoma experience late effects from their treatment, including second malignancies, cardiovascular and pulmonary disease, endocrine dysfunction, infertility and a compromised quality of life.
3,4,5,6,7,8,9,10,11 They are often more likely to die of second cancers or cardiopulmonary disease than of Hodgkin lymphoma, itself.
12,13 Today there are nearly 165,000 people alive in the United States with a history of Hodgkin lymphoma, underscoring the success of current treatment and the necessity of advancing therapeutic strategies that remain effective but have minimal adverse late effects.
BACKGROUND
In most developed countries, the incidence of Hodgkin lymphoma is bimodal by age, with an early peak seen in young adults and, later, a continuous rise after the age of 50 or 60 years. In less developed countries, the early peak is usually not apparent. These patterns reflect varying risk factors, etiologies, and host and tumor biology that contribute to important differences in the behavior of Hodgkin lymphoma in younger and older patients. Differences are also apparent by geographical region and socioeconomic groups, affecting both incidence and mortality rates. Internationally, there are nearly 70,000 new cases annually with wide variations in incidence ranging from similar to those in the United States to over 5.0 per 100,000.
14,15 New evidence related to the molecular biology
16,17,18,19,20,21 and etiology of Hodgkin lymphoma, such as heredity, Epstein-Barr virus (EBV), timing of oral exposure to the microbiome, and the nature of Th1 or Th2 responses, is beginning to support a consistent theoretical framework for the pathogenesis of Hodgkin lymphoma,
22,23,24,25,26,27,28,29,30,31,32 but a detailed discussion is beyond the scope of the chapter.
The malignant cells in classical Hodgkin lymphoma (cHL) (
Table 41-1) are called “Hodgkin” or “Reed-Sternberg” (H-RS) cells, depending on whether mononucleated or multinucleated.
33 In nodular lymphocyte predominant Hodgkin lymphoma, the characteristic malignant cell is referred to as the “lymphocyte predominant” (LP) cell.
33 Although the origin of malignant cells in Hodgkin lymphoma eluded investigators for many years, it is now apparent that H-RS cells most commonly derive from crippled germinal center B-cells that have engaged in an immune response but in which apoptosis has been blocked.
21,29,33,34,35,36 The relative rarity of H-RS cells, which aberrantly express antigens and are admixed with large numbers of normal cells that are, themselves, perturbed by heightened cytokine stimulation, made this identification especially difficult. LP cells are transformed germinal center B-cells. They have ongoing IgV mutations and express common B-cell antigens such as CD20.
16,32,37The different histologic subtypes of Hodgkin lymphoma
33 are associated with distinct clinical patterns (
Table 41-1). Nodular lymphocyte predominant Hodgkin lymphoma tends to present in peripheral nodes as early-stage disease. It tends to be indolent and responds very well to treatment, but may relapse quite late and, occasionally, transforms into diffuse large B-cell non-Hodgkin lymphoma.
38,39,40,41,42,43,44,45,46,47 Nodular sclerosis cHL, the most common subtype in young patients, shows a female preponderance and is primarily nodal in distribution, frequently involving the mediastinum. Mixed cellularity cHL, in contrast, occurs in a slightly older, predominantly male population and more likely at an advanced stage, often in the abdomen. Lymphocyte-depleted cHL, the subtype characteristic of the older patient, is frequently associated with B-symptoms
and is rarely localized.
33,48 Lymphocyte-rich cHL must be distinguished from the nodular lymphocyte predominant subtype.
33,41,49 It is seen in patients who are somewhat older but who often have limited stage disease that spares the mediastinum. Response to therapy and prognosis are excellent, and late relapses, such as in nodular lymphocyte predominant Hodgkin lymphoma, are infrequent. Although histopathologic subtype is a factor in determining outcome, therapeutic approach and prognosis, with some exceptions, depend primarily on stage.
Recognition of clinical stages extends back to 1902 when Dorothy Reed
50 distinguished between “first” and “second” stages of Hodgkin lymphoma. Her stages were not truly anatomical, but she recognized the tendency for spread to occur to “neighboring glands,” which is the foundation of modern staging. Surgical mapping of disease patterns during the era of staging laparotomy contributed to the understanding of patterns of spread and the refinement of stage-driven treatment.
51,52 Now, however, contemporary imaging techniques and the use of at least some chemotherapy in almost every patient make laparotomy rarely necessary. Clinical staging is sufficiently precise to permit selection of a therapeutic plan for most patients.
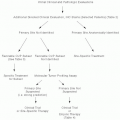
The emergence of
18 F-fluorodeoxyglucose positron emission tomography (FDG-PET) immensely improved the accuracy of staging and is now routinely included with CT scanning in the initial evaluation. FDG-PET more accurately delineates active disease sites, including those not identified or misidentified by CT scan, alone. Sequential FDG-PET scans have also become crucial in the assessment of response. The functional information provided by FDG-PET is critical to confirming persistent disease, especially in masses that remain after therapy.
53,54,55,56,57 Also, the rapidity of response as assessed by FDG-PET provides prognostic information. The resolution of abnormal hypermetabolic activity on an interim FDG-PET scan after only two or three
56,58,59,60,61,62 cycles of chemotherapy is independently associated with excellent progression-free and overall survival, whereas persistently positive scans at these early reassessments are associated with a high rate of relapse even if the scan is negative after completing all therapy. Early restaging FDG-PET scans are increasingly being incorporated into clinical trials to make real-time management decisions in hopes they will improve patient outcomes and minimize acute and late toxicities by eliminating exposure to unnecessary treatments.
Staging of Hodgkin lymphoma is currently based upon the 1989 Cotswolds modification
63 of the original Ann Arbor staging system
64 (
Table 41-2). Clinical stage, more than any other factor, determines treatment selection and by delineating the
initial sites of involvement provides the baseline for subsequent assessments of response. Standardized staging also permits comparisons of results across clinical trials.
Other clinical and laboratory findings may supplement the histopathologic subtype and clinical stage, helping to identify favorable or unfavorable subsets of patients who might benefit from the use of either less therapy or more.
65,66,67,68,69,70,71,72 Some factors, such as age and gender, define the host while others reflect biologic characteristics of the malignancy or the host’s response to it. These include serum levels of lactate dehydrogenase, β
2-microglobulin, albumin, alkaline phosphatase, hemoglobin, erythrocyte sedimentation rate, tumor bulk, and the location or number of disease sites. In addition, studies of the malignant cell and its microenvironment using comparative genomic hybridization, gene-expression profiling, and immunohistochemistry, among other techniques are beginning to provide prognostic information as well as important biologic insights.
17,18,73,74,75,76,77,78,79With rare exceptions, such as tumor bulk, single prognostic factors are insufficient to substantially alter initial treatment strategies for individual patients. However, aggregate prognostic factors do assist in assessing prognosis and selecting uniform subsets of patients for clinical studies. The International Prognostic Score (IPS), derived from an analysis of 4,695 patients with advanced Hodgkin lymphoma by the German Hodgkin Study Group (GHSG), is the most commonly used supplemental prognostic tool.
66 All of the patients received “state-of-the-art” therapy and also had sufficient follow-up so that long-term outcomes could be adequately judged. Seven factors were identified, each with a similarly small, but definite effect on prognosis, freedom from progression (FFP) and overall survival (
Table 41-3). The IPS is the sum of factors present in a given patient. It predicted 5-year survival rates ranging from 90% when either 0 to 1 factors were present to 59% with 4 to 7. A relatively small number, however, were included in this highrisk group. FFP was 74% at 5 years in those with 0 to 2 factors, but only 55% in patients who had 3 to 7. The identification of a large group of newly diagnosed patients who have an extremely poor outcome continues to be elusive. Another scoring system, based on time to first relapse, clinical stage, and anemia at relapse, has been developed by the GHSG for use in relapsed patients.
80 However, there has been less uniformity in adopting this tool in clinical trials. It may be that newer, more sophisticated tests will do a better job of assessing prognosis in Hodgkin lymphoma. For example, higher numbers of tumor-associated macrophages were recently reported to more strongly correlate with shortened survival than the IPS.
77
Number of Factors |
Percentage of Patients (%) |
Freedom from Progression at 5 y (%) |
Survival at 5 y (%) |
0 |
7 |
84 |
89 |
1 |
22 |
77 |
90 |
2 |
29 |
67 |
81 |
3 |
23 |
60 |
78 |
4 |
12 |
51 |
61 |
≥5 |
7 |
42 |
56 |
From Hasenclever D, Diehl V. A prognostic score for advanced Hodgkin’s disease. N Engl J Med. 1998;339(21):1506-1514. |