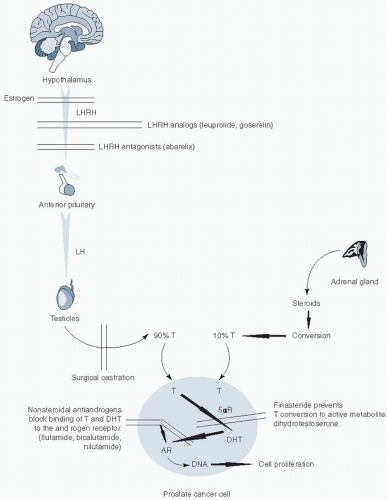
radiation as primary therapy, special consideration must be given to the reality that a significant portion of patients experiences recurrent disease. In a pooled analysis of men following radiation, 34% experienced a biochemical recurrence at 5 years,5 and similarly, rates of 15% to 31% have been reported following radical prostatectomy.6,7,8 Pound et al.7 reported that 34% of men with a rising PSA following radical prostatectomy developed metastatic disease at a median of 8 years, and once metastases were detected, patients experienced a median survival of 5 years. Investigators have identified that patients at diagnosis who have a PSA >20 ng per ml, Gleason score 8 to 10, or a clinical stage T2c or T3 are at high risk for recurrent disease following primary therapy.9 Learning from results in other solid tumors that even modestly active systemic chemotherapy can have curative potential when administered in the adjuvant setting, clinical investigators have studied the role of systemic therapy in conjunction with primary therapy for early stage disease with the hopes of improving outcomes.
Table 35-1 Prostate Cancer Staging | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
deprivation therapy is thought to result in superior outcomes based on RTOG 92-02 and EORTC 22961.17,18 However, some have criticized all of these studies in that they were designed several years ago, during which time the characteristics of prostate cancer patients and their management have changed. Currently, a smaller fraction of patients present with locally advanced disease than is represented in a number of these trials, and patients with bulkier disease are less likely to be cured with radiation alone. However, they may benefit more from the addition of androgen deprivation therapy than patients with less burdens of disease, skewing the results of these studies. Also, the total dose of radiation in many of the studies is on the order of 65 to 70 Gy, which is less than what is recommended today on the basis of dose escalation studies in prostate cancer.19,20,21 The proper timing and duration of androgen deprivation therapy as well as whether combined androgen blockade (CAB) is necessary is yet to be completely defined. Thus, until additional randomized trials demonstrate otherwise, androgen deprivation for a minimum of 3 years in conjunction with radiation is the standard of care for patients with high-risk disease.
Table 35-2 Phase III Trials Evaluating the Addition of Androgen Deprivation Therapy to External Beam Radiation | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
intermediate-risk disease (stage T2b, Gleason’s score 7, or PSA 10 to 20 ng per ml) should receive 4 to 6 months of neoadjuvant and concurrent androgen deprivation therapy, and the high-risk population (stage T2c or greater, or Gleason’s score 8 to 10, or PSA >20 ng per ml) should be offered at least 2 years of adjuvant hormonal therapy in addition to neoadjuvant and concurrent hormonal therapy.22 In the trials discussed above, adjuvant androgen deprivation largely consisted of luteinizinghormone releasing hormone (LHRH) agonist monotherapy, but other trials that have utilized three-dimensional conformal radiation have administered a LHRH-agonist in combination with an antiandrogen.23,24
target accrual. Immediate androgen deprivation therapy was associated with significantly less morbidity, but survival benefits were less clear.44,45 The Eastern Cooperative Oncology Group (ECOG) randomized men with nodal metastases at radical prostatectomy to immediate androgen deprivation therapy at the time of diagnosis or to delayed therapy until the time of disease progression. They noted an advantage in time to progression and survival with immediate treatment.12 The European Organization for Research and Treatment of Cancer (EORTC) noted a trend toward worse survival with a delay in treatment in a study that randomized men with node positive disease who did not undergo radical prostatectomy to either immediate or delayed androgen production.46 A meta-analysis also suggested improvements in progression-free survival (PFS) at 1 and 5 years as well as a small but significant survival advantage at 10 years in men who received immediate androgen deprivation.47
cancer-specific mortality and identify a population who should be considered for early androgen deprivation therapy. A PSA doubling time of <12 months following radical prostatectomy and <13 months following radiation therapy has been associated with prostate cancer-specific mortality.48,49 A PSA velocity of >2 ng per ml in the year prior to with radical prostatectomy or radiation has also been associated significantly with prostate cancer-specific mortality.50,51 Trials are needed to test PSA kinetics in a prospective fashion to further establish their validity in predicting outcomes.
Table 35-3 Standard Chemotherapy Regimens for Prostate Cancer | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
did not meet its primary endpoint, but an improvement in median survival from 16.6 months with placebo to 25.1 months with PROSTVAC-VF was noted.108 ECOG recently activated a phase II study with PROSTVAC in which men with mCRPC are randomized to 3 months of PROSTVAC vaccinations followed by docetaxel and prednisone or to immediate chemotherapy.
approach is the possibility of chemoresistant disease that may progress during neoadjuvant treatment, therefore resulting in a progression to a nonoperable stage because of delaying definitive local therapy. In addition, some patients may be exposed to unnecessary therapy based on inaccurate clinical staging.
Table 35-4 Bladder Cancer Staging | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree