Abstract
Understanding the development of specific components of the neonatal immune system is critical to the understanding of the susceptibility of the neonate to specific pathogens [1]. With the increasing survival of extremely premature infants, neonatologists and other physicians caring for these newborns need to be aware of the vulnerability of this population. Furthermore, it is important for neonatologists to be able to differentiate between immune immaturity and the manifestations of a true primary immunodeficiency that present during the neonatal period. Failure to properly identify primary or acquired immunodeficiency diseases can result in delayed diagnosis and treatment, adversely affecting outcomes. This chapter will briefly define the immune immaturity of the neonate and a diagnostic approach for primary immune deficiency diseases that may present in the neonatal period.
Understanding the development of specific components of the neonatal immune system is critical to the understanding of the susceptibility of the neonate to specific pathogens [1]. With the increasing survival of extremely premature infants, neonatologists and other physicians caring for these newborns need to be aware of the vulnerability of this population. Furthermore, it is important for neonatologists to be able to differentiate between immune immaturity and the manifestations of a true primary immunodeficiency that present during the neonatal period. Failure to properly identify primary or acquired immunodeficiency diseases can result in delayed diagnosis and treatment, adversely affecting outcomes. This chapter will briefly define the immune immaturity of the neonate and a diagnostic approach for primary immune deficiency diseases that may present in the neonatal period.
Immaturity of the Neonatal Immune System
The immaturity of a neonate’s immune response places them at an increased risk for serious infection. An understanding of the development of the neonatal immune system is essential in order to be able to differentiate the clinical manifestations of infections associated with immaturity from those that identify a specific acquired or primary immunodeficiency disease. For further detail on the development of the immune system, the reader should refer to Chapter 3. The primary components of immunity are subdivided into innate and adaptive responses. Innate immunity is antigen non-specific and composed of barriers, phagocytic cells, the complement system, pattern recognition receptors (PRRs), and soluble components of inflammation. Adaptive immunity, composed of T cells and B cells, provides immunologic specificity and memory. Because adaptive immunity is not fully developed in term and preterm infants, innate immunity plays a critical role in protecting the newborns from infection.
Innate Immunity
Barriers
Barriers provide the first line of defense from microbial invasion. Neonates have two important barrier regions: the mucosa (gastrointestinal and respiratory) and the skin. Each barrier possesses elements that reduce attachment and propagation of pathogens. The skin’s antimicrobial peptides are also present in high concentrations within vernix and amniotic fluid [2]. Lack of vernix and immature stratum corneum increases the risk of microbial invasion in premature infants. Medical procedures such as placement of intravenous lines breach cutaneous barriers and further raise the risk. Immediately after birth, the gastrointestinal (GI) microbiome is quickly established, providing barrier function based on the interaction between commensal organisms and host epithelium [3, 4]. Alteration of this homeostasis through hypoxic stress, sepsis, or use of antibiotics increases the risk for barrier dysfunction and resultant bacterial translocation [5].
Respiratory barrier defense include epithelial cells, resident phagocytes, mucociliary clearance, and the secretion of a number of proteins and peptides [6]. Surfactant proteins A and D play a valuable immune function by increasing opsonization of inhaled pathogens [7]. Respiratory mucosal function can be disrupted through altered mucus production, mechanical ventilation, surfactant deficiency, and chemical injury. The respiratory barrier defense is compromised in premature infants due to impaired mucociliary clearance, often due to deficiency in surfactant proteins, especially in infants with respiratory distress syndrome [8]. Unfortunately, these proteins are absent in commercially available surfactants due to destruction during the purification process [9].
Pathogen Recognition
Once the barrier function is breached, local sentinel immune cells respond, such as tissue macrophages that recognize invading pathogens via the activation of pattern recognition receptors (PRRs), including the toll-like receptors (TLRs), nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs), retinoic acid-inducible gene-I (RIG-I)-like receptors, and c-type lectin receptors (CLRs) [10, 11]. Present on multiple cell types, TLRs recognize extracellular and intracellular pathogens by their respective microbial products. The TLR ligand–receptor binding results in downstream production pro-inflammatory cytokines and chemokines that initiate antimicrobial effector mechanisms [12]. At present, there are 10 known TLRs in humans, and each receptor has a specific molecular activation trigger [12, 13]. Microorganisms often stimulate more than one TLR simultaneously with the result similar to a “molecular piano” playing “chords” signaling the presence of particular types of pathogens [13, 14]. TLRs play an essential role in pathogen recognition and response, thus alterations in their function can have consequences for host response. Most aspects of cytokine response are linked to TLR stimulation, which is diminished in neonates compared to children and adults [15–18].
The NOD-like receptors are involved in activating NF-κB and MAPK pathways, as well as in the formation of the inflammasome by mediating the activation of caspase-1 [19]. Mutation in the NLRP3 (NACHT domain-, leucine-rich repeat- and PYRIN domain-containing protein 3), a type NLR, leads to an autoinflammatory condition neonatal-onset multisystem inflammatory disorder (NOMID) that present in the neonatal period [20].
Adaptive Immunity
Adaptive immunity provides memory and specificity to the immune system and is mediated by T and B lymphocytes that recognize antigens in the context of their antigen specific receptors. Lymphocyte development begins in the bone marrow where a common progenitor either develops into a B cell within the bone marrow or migrates to the thymus to undergo T cell development. Both the T cell receptor (TCR) and B cells receptor (BCR) form via rearrangement of genes to form the antigen binding domains (complementary determining region 3 or CDR3) composed of variable (V), diversity (D), and joining (J) segments. The rearrangement of multiple VDJ segments is the first step in the generation of receptor diversity [21]. Further diversity is generated by terminal deoxynucleotidyl transferase (TdT) which inserts or deletes nucleotides at gene junctions creating further amino acid and CDR3 length variability. TdT is developmentally regulated throughout gestation, being less active in the fetus and premature infants resulting in less junctional diversity and shorter CDR3 lengths [22]. The impact of immature antigen receptors on immune priming and the TCR and BCR repertoire in preterm infants is unclear but likely plays an important role in the severity of intrauterine infections such as toxoplasmosis and cytomegalovirus (CMV) [23].
T-Cell Development
The primordial thymus develops at 6 weeks gestational age, and CD4+ and CD8+ T cells can be detected in the thymic cortex by 10 weeks. Hassall’s corpuscles in the thymic medulla, an indicator of T-cell selection, are evident by 14 weeks, and intact thymic architecture forms by 18 weeks. TCR rearrangement occurs during thymic development with the initial rearrangement of the D to J and then V to DJ segments of the TCRβ chain so that a naive T cell leaving the thymus have fully functional TCRs [24]. Naive T cells can be identified by distinct surface molecules, such as CD45RA, CCR7, CD31, and CD62L allowing their identification by flow cytometry [25]. In newborns, 80% to 90% of blood T cells express a naive phenotype. T-cell numbers and percentages are higher in newborns and infants compared to adults [26]. Normal newborn values for blood lymphocytes including T-cell subsets, B cells, and NK cells as measured by flow cytometry are shown in Table 5.1 [26].
| Subset | 0–3 months | 3–6 months | 6–12 months | 1–2 years | 2–6 years | 6–12 years | 12–18 years | adult |
|---|---|---|---|---|---|---|---|---|
| ALC (× 103/mm3) | 3,400–7,600 | 3,900–9,000 | 3,400–9,000 | 3,600–8,900 | 2,300–5,400 | 1,900–3,700 | 1,400–3,300 | 1,000–2,800 |
| CD3+ % | 73 (53–84) | 66 (51–77) | 65 (49–76) | 65 (52–75) | 66 (56–75) | 69 (60–76) | 73 (56–84) | 55–83 |
| CD3+ (× 103/mm3) | 3.68 (2.50–5.50) | 3.93 (2.50–5.60) | 3.93 (1.90–5.90) | 3.55 (2.10–6.20) | 2.39 (1.40–3.70) | 1.82 (1.20–2.60) | 1.48 (1.00–2.20) | 0.7–2.1 |
| CD19+ % | 15 (06–32) | 25 (11–41) | 24 (14–37) | 25 (16–35) | 21 (14–33) | 18 (13–27) | 14 (06–23) | 28–57 |
| CD19 (× 103/mm3) | 0.73 (0.30–2.00) | 1.55 (0.43–3.00) | 1.52 (0.61–2.60) | 1.31 (0.72–2.60) | 0.75 (0.39–1.40) | 0.48 (0.27–0.86) | 0.30 (0.11–0.57) | 0.3–1.4 |
| CD16+CD56+ % | 8 (04–18) | 6 (03–14) | 7 (03–15) | 7 (03–15) | 9 (04–17) | 9 (04–17) | 9 (03–22) | 10–39 |
| CD16+CD56+ (× 103/mm3) | 0.42 (0.17–1.10) | 0.42 (0.17–0.83) | 0.40 (0.16–0.95) | 0.36 (0.18–0.92) | 0.30 (0.13–0.72) | 0.23 (0.10–0.48) | 0.19 (0.07–0.48) | 0.2–0.9 |
| CD3+CD4+ % | 52 (35–64) | 46 (35–56) | 46 (31–56) | 41 (32–51) | 38 (28–47) | 37 (31–47) | 41 (31–52) | |
| CD3+CD4+ (× 103/mm3) | 2.61 (1.60–4.00) | 2.85 (1.80–4.00) | 2.67 (1.40–4.30) | 2.16 (1.30–3.40) | 1.38 (0.70–2.20) | 0.98 (0.65–1.50) | 0.84 (0.53–1.30) | |
| CD3+CD8+ % | 18 (12–28) | 16 (12–23) | 17 (12–24) | 20 (14–30) | 23 (16–30) | 25 (18–35) | 26 (18–35)> | |
| CD3+CD8+ (× 103/mm3) | 0.98 (0.56–1.70) | 1.05 (0.59–1.60) | 1.04 (0.50–1.70) | 1.04 (0.62–2.00) | 0.84 (0.49–1.30) | 0.68 (0.37–1.10) | 0.53 (0.33–0.92) | |
| CD4/45RA/62L+ % | 89 (61–94) | 88 (61–94) | 83 (58–91) | 79 (62–90) | 70 (50–85) | 58 (42–74) | 51 (31–65) | 7–31 |
| CD4/45RA/62L+ (× 103/mm3) | 2.25 (1.20–3.60) | 2.23 (1.30–3.60) | 2.10 (1.10–3.60) | 1.64 (0.95–2.80) | 0.96 (0.42–1.50) | 0.56 (0.31–1.00) | 0.39 (0.21–0.75) | 0.09–0.6 |
| CD8/45RA/62L+ % | 79 (56–88) | 77 (53–88) | 72 (47–87) | 71 (46–85) | 64 (42–81) | 58 (39–73) | 56 (42–73) | 6–19 |
| CD8/45RA/62L+ (× 103/mm3) | 0.73 (0.38–1.30) | 0.74 (0.45–1.20) | 0.70 (0.33–1.20) | 0.76 (0.40–1.40) | 0.54 (0.26–0.85) | 0.41 (0.20–0.65) | 0.30 (0.17–0.56) | 0.1–0.5 |
Note: Normal T, B, and NK values are presented as the 10th and 90th percentiles.
B-Cell Development and Immunoglobulin Production
As early as the 1st trimester, IgM+/IgD+ B cells develop and IgM antibody production begins. By the 15th week of gestation B cells are present in the spleen, blood, and lymph nodes. Immunoglobulin M responses to intrauterine infections occur by the 25th week. During mid-gestation, B cells exposed to antigen become anergic, as one mechanism of neonatal tolerance [23, 27]. Immunoglobulin production is dominated by polyreactive, low affinity, natural IgM [28]. Preterm and term infants can mount robust responses to proteins, known as T-dependent antigens, but cannot form protective antibody responses to T-independent polysaccharide bacterial antigens [29]. The majority of blood B cells are naïve, transitioning from the bone marrow to the germinal centers (GC). In the GC, they encounter antigen presenting cells and CD4+ T cells expressing CD40 ligand which induce cognate interactions with CD40 on B cells, resulting in isotype class switch [30]. All neonates have underdeveloped germinal centers, which contributes to the delay in class switch from IgM to IgA, IgG, and IgE [31]. Normal neonates are deficient in IgA, which may last throughout infancy.
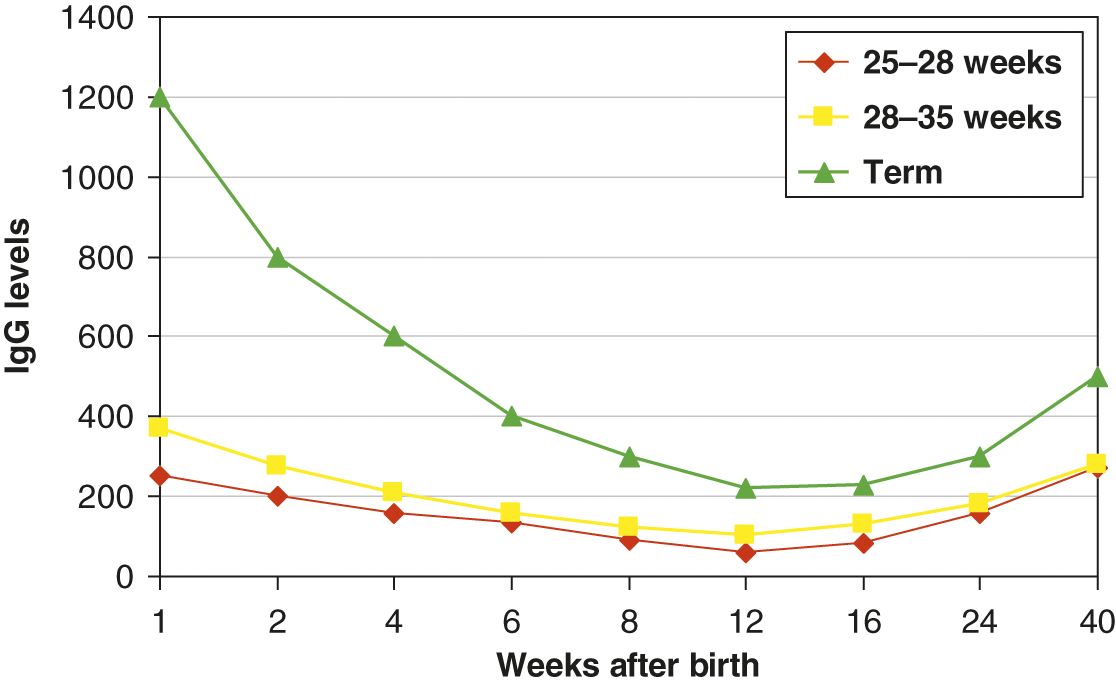
Infants acquire only IgG transplacentally. It is actively transported via the FcγRn in an energy dependent process primarily during the 3rd trimester, thus IgG levels are 10 to 20% higher in term infants compared to their mother [32]. Preterm infants have significantly lower IgG levels than their mothers due to insufficient/absent transplacental transport. IgG subclass distributions and pathogen specific antibody reflect maternal levels. Maternal IgG has a half-life of approximately 28 days and maternal levels often decline faster than the infant’s ability to generate their own IgG [33]. As a result, all infants have a transient hypogammaglobulinemia of infancy in which the IgG nadir occurs between 2 and 6 months of age. If an infant is premature, IgG levels can be less than 100 mg/dl. However, for the most part, these infants can mount robust IgM and IgG immune responses to pathogens or immunizations. The changes in IgG levels during the first year of life for preterm and term infants is illustrated in Fig. 5.1. Reference values for immunoglobulin isotypes for term and preterm (>28 weeks) infants are shown in Tables 5.2–5.4.
Fig. 5.1 Median IgG levels (y-axis) for term infants (triangle), preterm infants 28–35 weeks (square), and 25–28 weeks (diamond). Decay of maternal IgG over the first 40 weeks of life is shown on the x-axis.
Table 5.2 Plasma immunoglobulin levels from birth to 3 years of age
| Age | IgG (mg/dl) | IgA (mg/dl) | IgM (mg/dl) |
|---|---|---|---|
| Newborn | 598–1672 | 0–5 | 5–15 |
| 1–3 months | 218–610 | 20–53 | 11–51 |
| 4–6 months | 228–636 | 27–72 | 25–60 |
| 7–9 months | 292–816 | 27–73 | 12–124 |
| 10–18 months | 383–1070 | 27–169 | 28–113 |
| 2 years | 423–1184 | 35–222 | 32–131 |
| 3 years | 477–1334 | 40–251 | 28–113 |
Reproduced with permission from Buckley, et al. [195].
Table 5.3 Plasma immunoglobulin levels in preterm infants 25–28 weeks’ gesation
| Age | IgG* (mg/dl) | IgM* (mg/dl) | IgA* (mg/dl) |
|---|---|---|---|
| 1 week | 251 (114–552)** | 7.6 (1.3–43.3) | 1.2 (0.07–20.8) |
| 2 weeks | 202 (91–446) | 14.1 (3.5–56.1) | 3.1 (0.09–10.7) |
| 1 month | 158 (57–436) | 12.7 (3.0–53.3) | 4.5 (0.65–60.9) |
| 1.5 months | 134 (59–307) | 16.2 (4.4–59.2) | 4.3 (0.9–20.9) |
| 2 months | 89 (58–136) | 16.0 (5.3–36.1) | 4.1 (1.5–11.1) |
| 3 months | 60 (23–156) | 13.8 (5.3–36.1) | 3.0 (0.6–15.6) |
| 4 months | 82 (32–210) | 22.2 (11.2–43.9) | 6.8 (1.0–47.8) |
| 6 months | 159 (56–455) | 41.3 (8.3–205) | 9.7 (3.0–31.2) |
| 8–10 months | 273 (94–794) | 41.8 (31.1–56.1) | 9.5 (0.9–98.6) |
Reproduced with permission from Ballow M. et al. [33].
Notes: * Geometric mean.
** Normal ranges determined by taking antilog of (mean logarithm ±2 SD of the logarithms).
Table 5.4 Plasma immunoglobulin levels in preterm infants 29–32 weeks’ gestation
| Age | IgG (mg/dl)* | IgM (mg/dl)* | IgA (mg/dl)* |
|---|---|---|---|
| 1 week | 368 (186–728)** | 9.1 (2.1–39.4) | 0.6 (0.04–1.0) |
| 2 weeks | 275 (119–637) | 13.9 (4.7–41) | 0.9 (0.01–7.5)> |
| 1 month | 209 (97–452) | 14.4 (6.3–33) | 1.9 (0.3–12.0) |
| 1.5 months | 156 (69–352) | 15.4 (5.5–43.2) | 2.2 (0.7–6.5) |
| 2 months | 123 (64–237) | 15.2 (4.9–46.7) | 3.0 (1.1–8.3) |
| 3 months | 104 (41–268) | 16.3 (7.1–37.2) | 3.6 (0.8–15.4) |
| 4 months | 128 (39–425) | 26.5 (7.7–91.2) | 9.8 (2.5–39.3) |
| 6 months | 179 (51–634) | 29.3 (10.5–81.5) | 12.3 (2.7–57.1) |
| 8–10 months | 280 (140–561) | 34.7 (17–70.8) | 20.9 (8.3–53) |
Reproduced with permission from Ballow M. et al. [33].
Notes: * Geometric mean.
** Normal ranges determined by taking antilog of (mean logarithm ±2 SD of the logarithms).
Specific Immune Deficiency Disorders Presenting in the Neonatal Period
Primary immune deficiency diseases (PIDDs) represent over 300 genetic defects with distinct clinical phenotypes including susceptibility to infection, malignancy, immune dysregulation, and inflammation. The International Union of Immunological Societies (IUIS) Expert Committee classification system categorizes these disorders into common groups based on their pathogenesis, genotype, and clinical phenotypes [34]. The majority of PIDDs are not clinically apparent during the neonatal period, but a number of these disorders may manifest in early infancy. Due to the intrinsic immaturity of the immune system in preterm and term infants, it can be difficult to distinguish primary immune deficiency disease with underlying genetic defect from immune immaturity common to all newborns. Increased susceptibility to infections during the neonatal period is more likely the result of secondary immune deficiencies (immune immaturity, breakdown of innate barriers, and changes in microbial flora associated with prolonged use of antibiotics) and invasive medical procedures that compromise the barrier system (prolonged mechanical ventilation, indwelling urinary, venous, and arterial catheters) [35–37]. The IUIS classification system is shown in Table 5.5 and brief summary of disorders that present during the neonatal period are shown in Table 5.6. Specific conditions that often present during the neonatal period are described next.
Table 5.5 International Union of Immunological Societies classification of primary immunodeficiencies seen in the newborn period
|
|
|
|
|
|
|
|
|
Table 5.6 Clinical and laboratory findings of primary immune deficiencies seen in the newborn period
| Condition | Clinical/laboratory findings | Confirmation testing |
|---|---|---|
| Cellular immune deficiencies | ||
| ZAP70 deficiency | Low T-cell numbers with elevated CD4/CD8 T cell ratio | Absent mitogen response corrected ex vivo by phorbol ester. Genetic testing shows mutation in ZAP70 |
| Normal TREC | ||
| MHC-I deficiency | Sinopulmonary infections, granulomatous skin lesions. CD8 lymphopenia | CD8 lymphopenia on flow cytometry |
| Normal TREC | ||
| MHC-II deficiency | Diarrhea, hepatosplenomegaly, transaminitis, sclerosing cholangitis, pulmonary infections (PCP, encapsulated bacteria, herpesvirus), meningitis. CD4 T lymphopenia, hypogammaglobulinemia, absent germinal centers in lymph nodes | CD4 lymphopenia on flow cytometry |
| Normal TREC | ||
| DOCK8 deficiency | Eczema, food allergies, refractory viral skin lesions (HPV, HSV, VZV, molluscum), mucocutaneous candidiasis, pneumonias. Lymphopenia, eosinophilia, IgE may be elevated | DOCK8 mutation by genetic analysis |
| Low TREC | ||
| Ataxia–telangiectasia | Progressive cerebellar ataxia, oculocutaneous telangiectasia, recurrent sinupulmonary infections, low B and T cell numbers, elevated serum AFP and CEA, may have low IgA or IgG levels | ATM mutation by genetic analysis |
| TREC may be low | ||
| DiGeorge syndrome | Thymic hypoplasia/aplasia, hypocalcemia (parathyroid deficiency), congenital heart disease (conotruncal defects) | Meeting 2 of 3 clinical criteria (thymic hypoplasia/ hypoparathyroidism/ congenital heart disease), or detection of 22q11 deletion on FISH or microarray, or identification of known gene mutations (such as TBX1, CHD7) |
| Complete: Naive T cells < 50/mm3 + low/no mitogen proliferation | ||
| Partial: +/− low T cells, usually normal mitogen proliferation | ||
| May have low immunoglobulins if T cell defect severe | ||
| TREC may be low | ||
| Cartilage hair hypoplasia (CHH) | Short-limbed dwarfism, sparse hair, bone marrow failure, autoimmunity, thymic hypoplasia, Hirschsprung’s disease. Low to normal T cell numbers | RMRP mutation by genetic analysis |
| TREC may be low | ||
| Netherton syndrome | Generalized scaly erythroderma, “bamboo hair” (short brittle spiky), atopy (severe atopic dermatitis), elevated IgE, IgA, hypernatremic dehydration, failure to thrive, bacterial infections | SPINK5 mutation by genetic analysis. Hair microscopy confirms the presence of trichorrhexis invaginata for quick diagnosis |
| Normal TREC | ||
| Dyskeratosis congenita (DC) | Nail dystrophy, abnormal skin pigmentation, oral leukoplakia, severe enteropathy, recurrent infections, bone marrow failure. Lymphopenia, low B-cell numbers, hypogammaglobulinemia, decreased T-cell function possible | Telomere length analysis and genetic mutation identified in one of the multiple known mutations in addition to meeting clinical criteria |
| TREC may be low | ||
| NEMO deficiency syndrome | Conical teeth, sparse scalp hair, frontal bossing, absent sweat glands, eczema, recurrent bacterial infections or MAI infection. Low IgG, specific antibody deficiency, poor NK cell function, variable T and B cell dysfunction | IKBKG mutation by genetic analysis |
| Normal TREC | ||
| Antibody deficiency diseases | ||
| X-linked agammaglobulinemia | Recurrent bacterial infection, overwhelming enteroviral sepsis in newborn. Low or absent immunoglobulins | Lack of CD19 or CD20 on flow cytometry analysis. Genetic mutation in BTK |
| Normal TREC | ||
| Hyper-IgM syndrome | Infections with encapsulated bacteria, peri-rectal abscesses, oral ulcers, PCP pneumonia, Cryptosporidium infections, diarrhea (Salmonella, Giardia, Entamoeba), generalized lymphadenopathy and splenomegaly. Low or absent IgG, IgA, IgE, with normal or elevated IgM | Identification of known genetic mutations: CD40L, CD40, UNG, AID |
| Normal TREC | ||
| Diseases of immune dysregulation | ||
| Familial hemophagocytic lymphohistiocytosis (F-HLH) | Fever, splenomegaly, cytopenia, hypertriglyceridemia, hypofibrinogenemia, elevated sIL-2Rα, reduced NK cell function, evidence of HLH in BM or lymphoid tissues | Diagnostic criteria and/or Identification of known genetic mutations: PRF1, UNC13D, STX11, STXBP2 |
| Normal TREC | ||
| Wiskott–Aldrich syndrome | Eczema, thrombocytopenia, recurrent bacterial infections with low IgM, elevated IgA, normal IgG | WASP mutation by genetic analysis. |
| Normal TREC | ||
| Chédiak–Higashi syndrome (CHS) | Recurrent bacterial infections, oculocutaneous albinism, coagulation defects, neurological deterioration. Enlarged azurophilic lysosomal granules in neutrophils, eosinophils. Absent NK cytotoxicity, decreased neutrophil chemotaxis, neutropenia. Progress to accelerated phase (HLH) | Peripheral blood smear examination for pathognomonic giant cytoplasmic granules in leukocytes and platelets. Mutation in CHS/LYST by genetic analysis |
| Normal TREC | ||
| Griscelli syndrome type 2 | Partial albinism, neutropenia, thrombocytopenia, progressive neurologic deterioration. Progress to accelerated phase (HLH) | Microscopic examination of hair shaft showing clumps of pigment. No giant granules on peripheral smear (DDx CHS) |
| Normal TREC | ||
| XIAP deficiency | Recurrent splenomegaly, HLH triggered by virus, intractable IBD | Mutation in XIAP by genetic analysis |
| Normal TREC | ||
| Immunedysregulation, polyendocrinopahty, enteropaty, X-linked (IPEX) | Type I Diabetes in infancy, chronic enteropathy, failure to thrive, eczema, elevated IgA, and IgE | Absent T regulatory cells by FoxP3 staining, or mutations in the FOXP3 gene |
| Normal TREC | ||
| Defects in granulocyte function | ||
| Leukocyte adhesion deficiency (LAD) | LADI: Delayed separation of umbilical cord, impaired wound healing, serious/recurrent bacterial infections of skin and mucosal surfaces with absent pus formation. Persistent leukocytosis (>25,000 cells/uL) | LADI: Absence of CD11a, b, c, and/or CD18 expression on leukocytes on flow cytometry. Mutation in ITGB2 gene |
| LADII: Milder infections, no delay in separation of umbilical cord. Intellectual disabilities, short stature | LADII: Absence of H antigen on myeloid cells (Bombay), absence of CD15a expression on flow cytometry. Mutation in SLC35C1 gene | |
| LADIII: Similar to LADI plus life-threatening bleeding disorder | LADIII: Mutation in FERMT3 gene | |
| Normal TREC | ||
| Chronic granulomatous disease (CGD) | Recurrent bacterial and fungal infections with catalase positive organisms, inflammatory granuloma formation | Abnormal respiratory burst by DHR assay. Mutation in CYBB, CYBA, NCF1, NCF2, or NCF4 on genetic analysis |
| Normal TREC | ||
| Defects in Innate Immunity | ||
| Toll-like receptor signaling pathway deficiency (includes TLR3, UNC93B1, TRIF, TRAF3, TBK1) | HSV-1 encephalitis. Abnormal TLR function (performed when patient clinically well) | Mutation in TLR3 and associated genes |
| Normal TREC | ||
| NOMID | Frontal bossing, protruding eyes, saddle-shaped nose, recurrent fevers, migratory erythematous rash, impaired growth, chronic aseptic meningitis, sensorineural hearing loss, cerebral atrophy, lymphadenopathy, hepatosplenomegaly | NLRP3 mutation by genetic analysis |
| Normal TREC | ||
| Acquired immune deficiency | ||
| Pediatric AIDS | Opportunistic infections, inverted CD4/CD8 ratio, elevated IgA and IgM | Detection of HIV RNA in plasma or DNA in cells |
| Normal TREC | ||
Immunodeficiencies Affecting Cellular and Humoral Immunity
Cell-mediated immunity is pivotal to the neonatal immune response, but infants lack maternally acquired cellular immunity. T cell and combined immunodeficiency disorders commonly have their initial clinical manifestations during the neonatal period. Signs and symptoms suggestive of a disorder in cell-mediated immunity include opportunistic infections with organisms such as Pneumocystis carinii (jirovecii) pneumonia (PCP), Mycobacterium tuberculosis, fungal infections (most commonly due to Candida), disseminated viral infections, and graft-versus-host disease (GVHD) either due to maternally derived T cells or from transfusions with non-irradiated blood products. Manifestations of GVHD including macular erythematous rash, hepatitis, and chronic diarrhea can occur during the newborn period [38].
Severe Combined Immunodeficiency (SCID)
As implied in its name, without treatment, severe combined immunodeficiency (SCID) results in death due to infection or malignancy within the first few years of life. The condition is heterogeneous and are classified based on the relative impact on T, B, and natural killer (NK) cell numbers and function; (T−, B+, NK+), (T−, B+, NK−), (T−, B− NK+), or (T−, B−, NK−). The implementation of newborn screening using T-cell receptor excision circles (TRECs) have shown that the overall incidence of SCID and other cellular immune deficiencies in the United States is 1 in 20,000 live births with variation in incidence and type [39]. The different cellular phenotypes of SCID are summarized in Table 5.7. Because of the high complexity and rapid changes in the management of infants with T-cell deficiency and SCID, rapid referral to a tertiary care center is recommended for early hematopoietic stem cell transplantation (HSCT) prior to infections [40].
Table 5.7 Inheritance and distribution of lymphocyte subsets in different forms of SCID
| Lymphocyte subsets | Defect | Inheritance |
|---|---|---|
| T−/B−/NK− | ADA deficiency | Autosomal recessive |
| PNP deficiency (NK may be +) | Autosomal recessive | |
| Adenylate kinase 2 (Reticular Dysgenesis) | Autosomal recessive | |
| T−/B−/NK+ | RAG 1/2 | Autosomal recessive |
| Artemis | Autosomal recessive | |
| Cernunnos | Autosomal recessive | |
| Ligase IV | Autosomal recessive | |
| T−/B+/NK− | Common γc (X-linked SCID) | X-linked |
| Jak3 deficiency | Autosomal recessive | |
| T−/B+ /NK+ | IL-7Rα | Autosomal recessive |
| IL-2Rα | Autosomal recessive | |
| CD45 | Autosomal recessive | |
| Defects of CD3 chain (CD3δ, CD3ε, CD3ζ) | Autosomal recessive | |
| FOXN1 | Autosomal recessive |
T−, B+, NK+ SCID
Examples include IL-7Rα deficiency which results in intrathymic arrest in T-cell development and FOXN1 deficiency that results in abnormal thymic epithelia. Mutations in antigen receptor genes such as CD3δ, CD3ε, and CD3ζ, as well as CD45, a critical regulator of signaling thresholds, also lead to this phenotype [41]. In all cases, T cells fail to develop, but B cells and NK cells are spared [38].
T−, B+, NK− SCID
This phenotype results from abnormal signaling through the interleukin receptor common γ chain (γc) or further downstream defects involving JAK/STAT pathway. The X-linked form impairs the common interleukin γc cell surface receptor required for signaling of multiple cytokines including IL-2, IL-4, IL-7, IL-21, and IL-15. JAK-3 deficiency results in an identical phenotype because it associates with the common γc receptor for cell signaling, but is inherited in an autosomal recessive pattern [42]. Taken together, all are critical components of T, B, and NK cell function. Infants with these forms of SCID lack circulating T cells because T-cell maturation arrests in the thymus [43]. B cells are present in normal or increased numbers but are deficient in function. NK cell numbers and function are low as well. Gene therapy trials are underway to treat X-linked SCID.
T−, B−, NK+ SCID
The most common form of this SCID subtype is defects in recombinase-activating gene (RAG), which plays a critical role in the formation of T- and B-cell antigen receptors, leading to failure of T- and B-cell development, with intact NK cells [44]. Other deficiencies with similar phenotypes include Artemis (DCLRE1C) deficiency and DNA ligase IV deficiency (with microcephaly and facial dysmorphism), both involved in DNA repair, leading to defective V(D)J recombination of the antigen receptors. Defects in DNA repair enzymes carry the risk of radiosensitivity and malignancy as well as suppression of hematopoiesis. T- and B-cell development may not be totally arrested and “leaky” forms of this subtype often result in severe erythematous rashes and autoimmune cytopenia due to oligoclonal expansion of autoreactive lymphocytes.
T−, B−, NK− SCID
This subtype most commonly results from adenosine deaminase (ADA) deficiency. Reticular dysgenesis and PNP (purine nucleoside phosphorylase) deficiency also display similar lymphocyte distribution (reticular dysgenesis additionally impacts all hematopoietic lineages). ADA catabolizes the deamination of adenosine and 2′-deoxyadenosine, converting them to inosine [45]. Lack of ADA results in the intracellular accumulation of adenosine and 2′-deoxyadenosine, leading to the accumulation of deoxyadenosine triphosphate (deoxyATP), which has a feedback inhibition effect on ribonucleotide reductase, an enzyme required for normal DNA synthesis. Overall, DNA synthesis is impaired, and no lymphocyte cellular proliferation occurs with activation. In addition, 2′-deoxyadenosine is a cellular toxin reported to cause chromosome breakage and ultimately severe lymphopenia resulting in absent T cells, B cells, and NK cells. Enzyme replacement therapy with polyethylene glycol (PEG) modified bovine ADA given by subcutaneous injection once weekly has resulted in clinical and immunologic improvement [45–48]. Trials of gene therapy are currently underway to treat ADA deficiency, and an ex vivo gene therapy is approved in Europe [49].
Combined Immunodeficiency (CID)
Clinical phenotypes of CIDs are generally less profound and more variable in their manifestations than SCID. However, they carry similar risk for susceptibility to opportunistic infections and autoimmune cytopenias during the newborn period. Similar to SCID, different lymphocyte populations are affected and pathogenesis is variable [38]. For example, major histocompatibility complex (MHC) class-I or class-II deficiencies are associated with low levels of the CD8+ or CD4+ subsets, respectively. ZAP-70 deficiency results in a defective T-cell receptor signaling cascade resulting in poor proliferation to mitogens with normal numbers of CD3+ and CD4+ T cells but low numbers of CD8+ T cells. DOCK8 deficiency is characterized by low B and NK cell numbers, lymphopenia, elevated IgE, low IgM, eosinophilia, severe atopic disease, and recurrent staphylococcal and viral infections.
Combined Immunodeficiency with Associated Syndromic Features
Conditions that affect cellular and humoral immunity but have clearly recognizable clinical phenotypes can involve single or multiple genes. The majority have multiple organ systems involved beyond the immune system, and immune dysfunction may be a minor component of the syndrome. Representative examples seen in the newborn period are listed below.
Wiskott–Aldrich Syndrome (WAS)
The triad of immunodeficiency, thrombocytopenia, and eczema characterizes WAS. It is an X-linked recessive disorder associated with mutations in the WAS gene located in Xp11.23 encoding a protein known as Wiskott–Aldrich syndrome protein (WASp). Expressed by hematopoietic cells, this gene stabilizes actin polymerization and cytoskeleton arrangement. Multiple mutations have been identified resulting in different phenotypes (as in X-linked thrombocytopenia) within the syndrome but all share features indicating abnormal WASp function [50–55]. Platelets and lymphocytes have abnormal size, shape, and function. In particular, platelets are small, aggregate poorly, and are sequestered and destroyed in the spleen. Hemorrhage is the most common complication of WAS in infancy, and a major cause of morbidity and mortality [56]. T cells are also small with abnormal cytoskeleton leading to skewing toward a Th2 cytokine profile. There is a characteristic immunoglobulin profile of elevated IgA and IgE levels, with low IgM levels. Clinical manifestations that can be seen in early infancy include eczema, autoimmune hemolytic anemia, and thrombocytopenia. There is increased susceptibility to serious viral infections including disseminated herpes simplex, varicella-zoster, and molluscum contagiosum, as well as severe infections with encapsulated bacteria [34, 57, 58]. Infants with WAS may also experience an accelerated phase, with features of hemophagocytic lymphohistiocytosis (HLH, see the subsection on HLH later). Because of the long-term complications of immunodeficiency and malignancy, bone marrow transplantation is commonly applied to WAS and gene therapy is in development.
Ataxia–Telangiectasia
Ataxia–telangiectasia (AT) is an autosomal recessive disorder caused by biallelic mutations in DNA repair enzyme ATM (ataxia–telangiectasia mutated), a serine/threonine kinase encoded on chromosome 11q22.3, leading to DNA repair defects affecting all nucleated cells in the body [59]. Characteristic clinical features include progressive cerebellar ataxia as well as oculocutaneous telangiectasia that is rarely evident in the newborn. However, the enzyme defect also causes thymic hypoplasia which may result in abnormal TREC levels in the newborn screen due to low naive T-cell numbers [60]. Other laboratory manifestations include elevated serum α-fetoprotein and carcinoembryonic antigen, low B- and T-cell numbers, and selective IgA deficiency [61]. A subset of patients has hyper IgM phenotype with hypogammaglobulinemia and high or normal IgM levels [60]. Overall, immunodeficiency in AT is combined, remains low over time, and is not progressive [62]. Radiation sensitivity increases risk for lymphoreticular malignancies so early recognition is critical in clinical management. Treatment is based on disease manifestations. Prophylactic antibiotics may be used for recurrent sinopulmonary infections, and immune globulin replacement therapy can be considered for those with hypogammaglobulinemia or impaired specific antibody production.
The 22q11 Deletion Syndrome (DiGeorge Syndrome, DGS)
The combination of thymic defects with varying degrees of T-cell deficiency and associated congenital conotruncal cardiac defects, facial dysmorphism, cleft palate, and hypoparathyroidism are typical of DGS [63]. This syndrome is relatively common affecting up to 1 in 1,200 individuals [64, 65]. Abnormal migration of neural crest cells forming the third and fourth pharyngeal arches during early gestation is postulated to impair embryonic development of the thymus, parathyroid gland, heart, and face. Approximately 90% of patients with DGS have an underlying microdeletion within chromosomal region 22q11.2 but genetic deletions in chromosomes 10p13 and 17p13 are also associated with similar phenotypes [66, 67]. Within 22q11 deletions, T-box transcription-factor family, TBX1, has been implicated as a cause of most of the clinical manifestations [68, 69]. While most 22q11 deletions are sporadic, the deletion is inherited as an autosomal codominant condition; half of the siblings may be affected, and 10% of affected children have an affected parent. Conotruncal cardiac defects, including truncus arteriosus, tetralogy of Fallot, interrupted aortic arch type b, or aberrant right subclavian artery are the most frequent cardiac features. Dysmorphic facial features include prominent nasal root with bulbous tip, protuberant ears, cup-shaped helices, and small, carp-like mouth. Cleft lip and palate and velopharyngeal insufficiency result in speech abnormalities. Hypoparathyroidism leads to hypocalcemia, often associated with neonatal tetany and seizures. Developmental and language delay, learning disabilities, and neuropsychiatric problems including schizophrenia are common [63, 67, 70]. Other genetic syndromes associated with thymic aplasia include CHARGE syndrome most commonly associated with mutations in chromodomain helicase DNA-binding protein 7 (CHD7) gene on chromosome 8q12 or mutations in semaphorin-3E gene (SEMA3E) on chromosome 7q21 [71, 72]. Clinical manifestations of CHARGE include coloboma of the eyes, heart anomalies, choanal atresia, CNS abnormalities, developmental delay, and genital, and ear malformations [73].
Arrested T-cell development due to thymic aplasia or hypoplasia is a hallmark of the immune defects as the thymus maybe absent or reduced in size based on imaging. When T cells are completely absent (naive T cells < 50 cells/µl, termed complete DiGeorge syndrome) infection risk is extremely high and treatment requires thymic transplantation. With low CD3+ T counts less than 500/µL, there can be a poor proliferation response to T-cell mitogens, elevated CD4 to CD8 T cell ratios, and low numbers of naive T cells. Partial thymic hypoplasia is associated with antibody abnormalities such as poor response to immunizations, selective IgA deficiency, hypogammaglobulinemia, and autoimmunity (primarily autoimmune cytopenias) [74].
The treatment for individuals with DGS usually involves prophylaxis alone as most patients have the partial form and show a gradual improvement in T-cell development. Treatment for complete DGS involves intravenous immunoglobulin (IVIg) and thymus transplantation [75]. Thymic tissues obtained during elective cardiac surgery can be implanted, and this has been successfully carried out without human leukocyte antigen (HLA) matching or the risk of GVHD [75]. Otherwise, treatment is focused on correction of the cardiac and parathyroid defects and prevention of opportunistic infections with prophylactic antibiotics.
Cartilage Hair Hypoplasia (CHH)
Cartilage hair hypoplasia is characterized by short-limbed dwarfism with metaphyseal dysostosis, sparse hair, bone marrow failure, autoimmunity, and neuronal dysplasia of the intestine. Affected individuals are susceptible to developing lymphoma and other cancers [76]. It is also associated with varying degrees of thymic hypoplasia ranging from SCID to normal thymic output, thus infants with this condition may have abnormal newborn screen with low TRECs. Mutation in RMRP gene is responsible for the condition [76]. CHH presenting with SCID require HSCT. HSCT corrects immune deficiency and autoimmunity but does not improve the musculoskeletal or growth features. Careful monitoring of bowel function during the first year of life is important as Hirschsprung’s disease is common.
Netherton Syndrome
Netherton syndrome is a rare autosomal recessive skin disorder caused by mutations in the serine protease inhibitor of Kazal type 5 gene (SPINK5) located on chromosome 5q32 [77, 78]. Typical presentation is characterized by the classic triad of congenital ichthyosiform erythroderma, specific hair shaft abnormality termed trichorrhexis invaginata (“bamboo hair”), and atopy (severe atopic dermatitis, elevated serum levels of IgE, and later development of asthma and hay fever) [79]. Infants with this condition typically present at birth with generalized scaling erythroderma, and have life-threatening complications such as hypernatremic dehydration (from water loss through the dysfunctional skin barrier), failure to thrive, and bacterial infections. IgE and IgA are elevated and other immunoglobulins may be decreased [78]. These infants should be managed in an intensive care unit, with careful monitoring of body temperature, fluid, and electrolytes, as well as prevention and treatment of infections. Dermatitis should be managed with good skin care with liberal application of moisturizers, topical anti-inflammatory agents (corticosteroids, calcineurin inhibitors), and oral antihistamines for the management of pruritis.
Dyskeratosis Congenita (DC)
Dyskeratosis congenita is characterized by nail dystrophy, abnormal skin pigmentation (poikiloderma), and oral leukoplakia. Individuals with DC are predisposed to cancers and bone marrow failure. Some may present with palmar hyperkeratosis, pancytopenia, intrauterine growth retardation (IUGR), sparse scalp hair and eyelashes, recurrent infections, developmental delay, and in severe cases, cerebellar hypoplasia, microcephaly, and organ fibrosis (pulmonary fibrosis, esophageal stricture, liver cirrhosis) [80]. Immune phenotypes are variable, but lymphopenia, low B-cell numbers, hypogammaglobulinemia, and decreased T-cell function are frequently found. Onset of disease in infancy is associated with more severe immunologic and somatic features, especially severe enteropathy [81]. It is a condition of defective maintenance of telomeres resulting in shortened telomere length. Various inheritance patterns have been observed depending on the affected gene, including autosomal dominant, autosomal recessive, and X-linked. Treatment is based on clinical manifestations and ongoing screening is recommended for bone marrow failure, myelodysplastic syndrome, solid tumors, pulmonary fibrosis, liver disease, thyroid function, and osteopenia.
NEMO (NF-κB Essential Modulator) Deficiency Syndrome
NEMO deficiency syndrome is a rare X-linked recessive disorder characterized by anhidrotic ectodermal dysplasia with immunodeficiency (EDA-ID) caused by hypomorphic mutations in the IKBKG (inhibitor of nuclear factor kappa-B kinase regulatory subunit gamma) gene encoding NEMO protein, a key regulator of the canonical NF-κB signaling pathway. Loss-of-function mutations of IKBKG leads to X-linked incontinentia pigmenti, which is usually lethal in male fetuses. Hypomorphic IKBKG mutation leads to EDA-ID with diverse clinical manifestations [82]. Infants with NEMO deficiency present with abnormal development of ectodermal tissue including skin, hair, teeth, and sweat glands, along with severe infections. Typical EDA-ID presentation includes conical teeth, sparse scalp hair, frontal bossing, absence of sweat glands, along with early multiple and severe bacterial infections with pyogenic bacteria (Streptococcus pneumoniae, Staphylococcus aureus, Haemophilus influenzae) or mycobacteria (Mycobacterium avium) [83]. Infants may present with eczematous dermatitis soon after birth. Clinical manifestations may be evident soon after birth and their life expectancy depends on the degree of immune deficiency. Immunologic manifestations include hypogammaglobulinemia, some with hyper IgM, impaired specific antibody production, impaired T-cell receptor activation, monocyte dysfunction, poor cytotoxic NK cell activity, and variable T and B cell dysfunction (such as low naive T cells, low memory CD27+/CD19+ B cells) [82, 84]. Treatment includes immune globulin replacement therapy and antimycobacterial prophylaxis. Chronic herpes antiviral prophylaxis may also be used in those with herpesvirus infections. HSCT is recommended for those with severe disease course.
Predominantly Antibody Deficiencies
Clinical Manifestations of Disorders in Antibody Production
Deficiencies in B-cell function are difficult to recognize during the newborn period due to passive acquisition of maternal IgG and low IgA levels throughout infancy. Defects in antibody production are characterized by bacterial sinopulmonary infections, meningitis, and sepsis as well as persistent enteroviral infections of the gastrointestinal tract or central nervous system [37]. Catabolism of passively acquired antibody occurs with a half-life of approximately 30 days, although maternal antibody can be detected throughout the first eighteen months of life [6]. As the levels of maternal antibody decline, immunoglobulin deficiency in the neonate becomes apparent and generally can be detected after age six months, or even earlier in preterm infants.
Transient Hypogammaglobulinemia of Infancy (THI)
The question remains as to whether THI is a true primary immune deficiency or an extension of the physiologic nadir that occurs as maternal antibody disappears and the infant begins its own antibody production [85]. Normal IgG nadir occurs between 3 and 6 months in term infants with significantly lower values seen in premature infants [33]. In THI, IgG levels are greater than two standard deviations below normal age-related ranges and all immunoglobulin isotypes can be affected (see Tables 5.2–5.4) [85–87]. This phenomenon should be considered in chronically ill infants who remain hospitalized for several months, as recurrent infection, malnutrition, and repeated phlebotomy accentuate antibody loss. Examination of the impact of low serum gammaglobulin (levels <4 g/L total serum IgG) in preterm infants (24–32 weeks gestational age) revealed an increased risk for infection but not mortality compared to those with levels >4 g/L after controlling for gestational age, and the risk did not decrease with use of IVIg [88, 89]. There is evidence that infants with THI have a transient defect in CD4+ T helper cell function when compared to normal infants [90]. However, B-cell numbers in these children are normal and they are able to generate specific antibody responses following immunization with T-dependent antigens such as tetanus toxoid [89].
X-Linked Agammaglobulinemia (XLA)
XLA results in profoundly diminished levels of all immunoglobulin isotypes that remain depressed for life. The defect in XLA is due to a mutation in the gene encoding B cell-specific src-associated tyrosine kinase (Bruton’s tyrosine kinase or BTK). This protein plays a pivotal role in early B cell development at the pre-B-cell stage in the bone marrow leading to absent B cells in peripheral blood and lymphoid tissues [91]. Lack of circulating CD19 and CD20 B cells allows the condition to be diagnosed by flow cytometry analysis [92]. T-cell development, numbers, and function are normal. Clinical manifestations that have been observed in newborns include overwhelming enteroviral sepsis [93]. Treatment for XLA is immune globulin replacement therapy.
Hyper-IgM (HIGM) Syndrome
In the most common form of HIGM syndrome, a molecular defect in immunoglobulin class-switch results from a mutation in CD40 ligand (CD40L) which mediates class switch through cognate interaction between CD40L on the T cell and CD40 expressed on B cells. There are low or absent levels of serum IgG, IgA, and IgE with normal to elevated levels of IgM [94]. During the neonatal period, immunoglobulin profiles can appear very similar to those of normal infants or infants with an intrauterine infection. B-cell numbers are normal but infections with encapsulated organisms are common. More than half of the infants with hyper-IgM syndrome have neutropenia with associated peri-rectal abscesses and oral ulcers [95]. In addition, pneumonitis due to infection with P. jirovecii is frequent and cholangitis due to Cryptosporidium has been reported. There is an increased susceptibility to diarrhea due to Salmonella, Giardia, and Entamoeba [96]. The disorder has also been reported in association with congenital rubella. Nodular lymphoid hyperplasia of the intestinal tract and generalized lymphadenopathy and splenomegaly can occur in young infants [96]. HIGM is most commonly inherited as X-linked primary immune deficiency but autosomal recessive forms have also been recognized [95, 97]. The mutation in the CD40LG gene in the X-linked form has been mapped to Xq26 [97, 98]. Other rare molecular defects that present with hyper-IgM syndrome phenotype include CD40 deficiency (mutations in CD40 gene, inherited AR), AID deficiency (mutations in AIDCA gene, AD, or AR), UNG deficiency (mutations in UNG gene, AR), and more recently identified mutations in PIK3CD and PI3KR1 genes [95, 99, 100].
All patients with HIGM are treated with immune globulin replacement therapy. Additional therapies depend on the type of hyper-IgM and the disease manifestations of each patient. PCP prophylaxis should be considered for those with CD40L and CD40 deficiency as these defects lead to combined immunodeficiency [96]. G-CSF (granulocyte-colony stimulating factor) may be considered for those with severe chronic neutropenia [101]. HSCT is the only curative approach for patients with CD40L deficiency and early transplant may prevent liver disease [102].
Antibody Deficiency Associated with Secondary Immune Disorders
Many conditions seen in neonates accompany antibody deficiency as part of their clinical spectrum. Specifically, Turner syndrome is often associated with decreased IgG and IgM levels [103]. Immunodeficiency, centromeric heterochromatin, and facial abnormalities (ICF) syndrome results in the instability of chromosomes 1, 9, 16, and 20 with associated findings of low total immunoglobulin levels, absent isohemagglutinins, and dysgammaglobulinemia [104, 105]. Hypogammaglobulinemia is a common associated finding in Trisomy 21, Monosomy 22, Trisomy 8, and Chromosome 18q-syndrome [106]. Not surprisingly, sickle cell disease and congenital asplenia syndromes have increased susceptibility to sepsis due to encapsulated bacteria [107]. The functional asplenia observed in sickle-cell disease actually begins during the first year of life. Congenital infections with rubella, CMV, HIV, Epstein–Barr virus (EBV), and toxoplasmosis can impair both antibody production and function [6]. Antibody deficiency can occur due to protein losing enteropathies and any other conditions that result in excessive loss of protein. Low total IgG levels at birth in the term infant suggests a maternal antibody deficiency disease [108].
Diseases of Immune Dysregulation
Hemophagocytic Lymphohistiocytosis (HLH)
Hemophagocytic lymphohistiocytosis is characterized by immune dysregulation with hyperinflammatory response associated with aberrant activation of macrophages and lymphocytes leading to overwhelming cytokine release. It typically affects infants from birth to 18 months of age. Infants may present in the newborn period with a constellation of symptoms including persistent fever, splenomegaly with cytopenia, hypertriglyceridemia, and hypofibrinogenemia. Other common laboratory findings include elevated ferritin and sIL-2Rα levels, reduced NK cell function, elevated sCD163, and elevated granzyme B. B- and NK cell numbers may be decreased with normal T-cell numbers and variable immunoglobulin levels. Evidence of Hemophagocytosis in the bone marrow on liver biopsy confirms the diagnosis. The syndrome can be divided into two subtypes: primary or familial HLH and secondary HLH [109–112]. Primary forms are most common in the newborn period.
Familial HLH (FHL)
Familial HLH is associated with inherited gene mutations, categorized into five subtypes (FHL 1–5) and identified mutations to date include mutations of PRF1 (encoding perforin, in type 2 FHL), UNC13D (Munc13-4, type 3), STX11 (syntaxin 11, type 4), and STXBP2 (Munc18-2, type 5). In type 1 FHL, a gene mutation has not been identified. These gene mutations affect T and NK cell functions related to target cell cytolysis (target cell pore formation, membrane fusion, intracellular transport, release of cytotoxic granules) [109–111].
Management of acutely ill HLH patients with deteriorating organ function include HLH-specific treatment with dexamethasone and etoposide, with the most commonly used protocol being HLH-94 [113]. Intrathecal chemotherapy (such as methotrexate, hydrocortisone) may be used for patients with central nervous system (CNS) involvement.
There are primary immunodeficiency diseases associated with HLH, and some of these conditions may present in the neonatal period, including Wiskott–Aldrich Syndrome (described earlier), Chédiak–Higashi syndrome, Griscelli syndrome type 2, and X-linked inhibitor of apoptosis (XIAP) deficiency, described next [109, 112].
Chédiak–Higashi Syndrome (CHS)
Chédiak–Higashi syndrome is a rare autosomal recessive condition of aberrant lysosomal trafficking characterized by recurrent bacterial infections (neutrophil defects), oculocutaneous albinism (OCA, hypopigmented skin, hair, and eyes), mild coagulation defects, and progressive neurological deterioration. The diagnosis of CHS can be made by examining peripheral blood smear for pathognomonic giant cytoplasmic granules in leukocytes and platelets. A mutation in the CHS1/LYST gene at 1q42.1–2 is responsible for the condition [114]. In 85% of cases, affected individuals with CHS develop an accelerated phase consistent with HLH. Once individuals with CHS enter the accelerated phase, prognosis is poor. Early diagnosis and hematopoietic stem cell transplantation (HSCT) corrects the hematologic and immunologic defect, but does not prevent development of neurological deterioration in adolescence and early adulthood [115]. Treatment includes prophylactic antibiotics to prevent infections, G-CSF to correct neutropenia, and interferon gamma. HSCT corrects immunologic and hematologic manifestations, but does not prevent neurologic deterioration or oculocutaneous albinism [116].
Griscelli Syndrome Type 2 (GS2)
Griscelli syndrome type 2 is a rare autosomal recessive condition characterized by partial albinism, neutropenia, thrombocytopenia, and progressive neurologic deterioration thought to be due to cerebral lymphohistiocytic infiltration, and development of accelerated phase (HLH, only in type 2). GS2 results from a mutation in RAB27A, a GTP-binding protein involved in cytotoxicity and cytolytic granule exocytosis of lymphocytes [117]. Diagnosis can be made via microscopic examination of the hair shaft showing clumps of pigment. The absence of giant granules and the histologic characteristics of hypopigmentation differentiate this condition from CHS. Griscelli syndrome should be considered in infants with silvery-gray hair, hepatosplenomegaly, and immunodeficiency [118].
X-Linked Inhibitor of Apoptosis (XIAP) Deficiency
XIAP deficiency is a rare X-linked immunodeficiency that can affect boys during early infancy. Mutations in the XIAP gene at Xq25 leads to the condition that resembles familial HLH [119]. The most frequent clinical manifestations are HLH, recurrent splenomegaly, and to a lesser extent, IBD (often intractable) [120, 121]. EBV often triggers HLH, but HLH can also be triggered by CMV, HHV-6 infections, or even in the absence of a documented infection. Splenomegaly may be the initial clinical presentation of the disease in many cases.
Secondary HLH
Secondary HLH is usually associated with infectious diseases (most commonly with EBV), autoinflammatory and autoimmune diseases (more commonly known as macrophage activation syndrome), malignancy, HIV infection, hematopoietic stem cell or organ transplantation, immunosuppression, or metabolic diseases.
IPEX Syndrome
Immunodysregulation, polyendocrinopathy and enteropathy, X-linked (IPEX) syndrome is a primary immunodeficiency that can present in the newborn period and can even be diagnosed by fetal ultrasound [122–124]. This condition is commonly (60%–90%) associated with a mutation in the forkhead box protein 3 (FOXP3) gene located on the X chromosome (125, 126]. FOXP3 is a DNA-binding protein that functions as a transcriptional repressor necessary for the development of TREG, which in turn is responsible for regulating other immune cell functions including peripheral immunologic tolerance [127]. TREG in infants with IPEX syndrome exhibit impaired suppressor function as well as decreased IL-2 and IFN-γ production by peripheral blood mononuclear cells following activation [128]. Although mutations in the FOXP3 gene are associated with IPEX syndrome, FoxP3 protein expression does not appear to correlate with disease severity [123]. Clinical manifestations include autoimmune enteropathy (severe watery diarrhea and failure to thrive), eczematous dermatitis, and type I diabetes mellitus, which can all present during the neonatal period. In addition, a chronic inflammatory state is observed due to excessive cytokine production and autoantibody formation [129, 130]. The diagnosis of IPEX syndrome is primarily based on clinical signs. Laboratory findings may include elevated serum IgE, autoantibodies to pancreatic islet antigens, thyroid antigens, or small bowel mucosa, autoimmune cytopenias (anemia, thrombocytopenia, and/or neutropenia), intermittent eosinophilia, and decreased numbers of FoxP3-expressing T cells in peripheral blood [123]. Other serum immunoglobulins (IgG, IgA, IgM) and levels of complement are normal, as are circulating leukocyte counts including neutrophils, T cells, and B cells [131]. Treatment involves immune suppression with tacrolimus or sirolimus and HSCT.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree