Abstract
Ancient concepts of the blood were described by Hippocrates and Galen 2000 years ago in their doctrine of “humors.” It was believed that the body was made up of four humors – blood, phlegm, black bile, and yellow bile – and that these four components had the qualities of heat (hot-blooded!), cold, moist, and dry. The Galenic concept of the blood prevailed through the Middle Ages. Health or disease were a result of an imbalance, between these humors. This was the basis of the practice of therapeutic bloodletting (which, fortunately, was performed infrequently on children) through the mid nineteenth century as a way to rid the body of the imbalance of humors believed to cause a wide variety of diseases.
Ancient concepts of the blood were described by Hippocrates and Galen 2000 years ago in their doctrine of “humors.” It was believed that the body was made up of four humors – blood, phlegm, black bile, and yellow bile – and that these four components had the qualities of heat (hot-blooded!), cold, moist, and dry. The Galenic concept of the blood prevailed through the Middle Ages. Health or disease were a result of an imbalance, between these humors. This was the basis of the practice of therapeutic bloodletting (which, fortunately, was performed infrequently on children) through the mid nineteenth century as a way to rid the body of the imbalance of humors believed to cause a wide variety of diseases.
The hematology of the fetus and newborn is a relatively recent area of study whose development depended upon the evolution of the science of hematology and, especially, upon methods to study the blood and its elements. As Wintrobe has pointed out, the development of the field of hematology has been driven by technology. He divided the evolution of hematology into two general areas: morphology, which relied on the development of microscopy, and quantitation of the elements of the blood, which came later [1].
The invention of the microscope enabled identification of the blood cells. Antonie van Leeuwenhoek, working in Delft, Holland, constructed a primitive microscope from the minute biconcave lens mounted between two metal plates attached to a screw that permitted focusing. Leeuwenhoek’s publication in 1674 contained the first accurate description of the red blood corpuscles [2]:
The blood is composed of exceedingly small particles, named globules, which in most animals are red in color … These particles are so minute that 100 of them placed side by side would not equal the diameter of a common grain of sand.
In the centuries following, the development of compound microscopes with two lenses greatly increased magnification and minimized spherical aberration, permitting more accurate descriptions of the blood cells. Dr. William Hewson, who has been designated as one of the “fathers of hematology,” noted that the red cells were flat rather than globular and also described the leukocytes for the first time [3]. The last of the formed elements of the blood, the platelet, was recognized independently by several investigators. The most definitive early work on the platelet was done by Giulio Bizzozero. His monograph, published in 1882, clearly recognized these cells as being distinct from red and white blood cells, and suggested that they should be called Blutplättchen. He also assigned a hemostatic function to the platelet [4]. Dr. William Osler, early in his illustrious career, also described platelets accurately, although he believed that they may be infectious agents, perhaps analogous to bacteria [5].
With improvements in microscopy, the morphology of the fixed blood cells began to be examined using thin films of blood, spread and dried on glass slides, which were then stained with aniline dyes that stained differentially the nuclei and granules of the leukocytes. Staining of peripheral blood smears was developed by Paul Ehrlich in 1877, while he was still a medical student [6], and became practical in the early twentieth century by the work of Dr. James Homer Wright of Boston, who formulated the polychromatic Wright stain that is still used today for morphologic examination of the blood and bone marrow. The development of supravital dyes provided a method for assessment of erythropoiesis by reticulocyte counts. These techniques permitted the flowering of morphologic hematology, and many blood diseases such as the leukemias and the various types of anemia were described on the basis of typical morphological findings.
Hematology as a quantitative discipline began with the development of practical and reliable methods to quantify accurately the numbers of the various blood cells. These methods used gridded chambers of uniform depth (hematocytometers) into which precisely diluted suspensions of blood were placed. The numbers of cells in the chamber were counted and, when combined with the known dilutions, the actual numbers of cells per cubic milliliter in the patient’s blood could be calculated. Hemoglobin levels were estimated by comparing the density of color in fixed dilutions of hemolyzed blood with colorometric standards and, later, by spectroscopy. For many years, hemoglobin values were reported as “% of normal”; and because the definition of “normal” was often different there was considerable variability from study to study. In 1929, Dr. Maxwell Wintrobe described his method for obtaining the hematocrit or packed red-cell volume (PCV) by centrifugation of blood in a glass tube [7]. He then defined so-called red-cell indices, the mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC), which proved of enormous value in classifying the various forms of anemia [8]. The latest advance in blood-cell quantitation began in the 1950s with the introduction of increasingly more complicated and sophisticated computer-driven electronic instruments that measure hemoglobin very accurately, the numbers of all the blood cells, as well as the red-cell indices and the red-cell distribution width (RDW). Some instruments now also provide automated differential counts of the leukocytes.
Most of the pre-twentieth century American pediatric textbooks gave scant attention to hematologic problems of the neonate. Dr. W.P. Dewees’s 1825 A Treatise on the Physical and Medical Treatment of Children, arguably the first American pediatric textbook, and Dr. Job Lewis Smith’s 1869 A Treatise on the Diseases of Infancy and Childhood gave only passing notice to blood conditions of the neonate, such as neonatal jaundice and hemorrhage from improper ligature of the umbilical cord [9, 10]. However, the monumental pediatric text of Dr. L. Emmett Holt, The Diseases of Infancy and Childhood, first published in 1897, contained a section on “the Diseases of the Newly-Born,” including the hemorrhagic disease, and a 17-page section on “the Diseases of the Blood,” which included the normal blood findings in the newborn [11]. Holt was obviously familiar with the many studies published in the German literature, and his descriptions are reasonably consistent with modern findings:
The percentage of hemoglobin is highest in the blood of the newly born … At this time the number of red blood corpuscles is from 4,350,000 to 6,500,000 in each cubic millimeter … In size, a much greater variation is seen in the red cells of the neonate. In the blood of the foetus there are present nucleated red corpuscles or erythroblasts. These diminish in number toward the end of pregnancy. These are always found in the blood of prematures, but in infants born at term, they are seen only in small numbers. The number of leukocytes in the blood of the newly born is three or four times that of the adult, being on the average 18,000 per cubic millimeter.
In 1921, Dr. W.P. Lucas and associates from the University of California Medical School in San Francisco described their extensive studies of the blood of 150 infants at birth and during the first 2 months of life [12]. Their samples were obtained from serial punctures of the longitudinal sinus! The polycythemia of the newborn and changes in the leukocytes were defined clearly.
In 1924, Dr. H.S. Lippman from the University of Minnesota published detailed studies of the blood of newborn infants [13]. He noted (without further details) that “Denis published the first observations on the subject in 1831.” Lippman’s review of the literature cited 70 previous articles on the hematology of the newborn. Most of these studies were published in European, especially German, journals. Although there was considerable variability because of different methods and standards, the consensus of these early studies was that “Hemoglobin values at birth are higher than at any other period in the children’s life.” Some of these studies described reticulocytosis and normoblastemia in the first day of life, which declined rapidly in the first week of life. Lippman conducted serial studies of capillary blood over the first 48 hours of life in 71 normal newborns as well as changes in the leukocytes during this period.
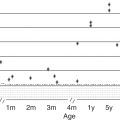
It has been known for 100 years that the red-blood cells (RBC) of the fetus and newborn are large compared with those of adults, as determined by microscopic measurement of red-cell diameter. Newer electronic cell-sizing techniques have demonstrated that the mean MCV of the neonate’s red blood cells averages 110 fl, compared with the 90 fl of adults. The red cells of midgestational fetuses are even larger [14].
In 1856, E. Korber, in his doctoral dissertation, is reported to have described his experiments that showed that solutions of the hemoglobin of newborn infants resisted denaturation by strong alkaline solutions and maintained a red color, while hemoglobin solutions from adults treated in the same way were rapidly denatured and decolorized [15]. The property of alkali resistance became the basis of the Singer one-minute alkali denaturation test for quantitation of fetal hemoglobin (HB F), as well as the Apt test, used to differentiate fetal from swallowed maternal blood in infants with gross blood in the gastrointestinal tract [16, 17]. Fetal hemoglobin is also resistant to acid denaturation, which is the basis for the red-cell acid elution staining procedure of Drs. C. Kleihauer, H. Braun, and K. Betke that is used widely to quantitate the magnitude of fetomaternal transfusions [18].
The understanding of the protein structure of hemoglobin advanced rapidly in the 1950s when it was shown that adult hemoglobin, Hb A (α2 β2), is a tetramer of alpha (α) and beta (β) polypeptide chains and that Hb F (α2 γ2) contains a different pair of polypeptide chains designated as gamma (γ) chains [19, 20]. During fetal development, synthesis of γ chains predominates, but with approaching term there is a fall-off of γ-chain synthesis and a simultaneous reciprocal increase in β-chain synthesis. The regulatory mechanisms that govern this “β/γ switch” remain to be elucidated. The blood of the newborn contains large amounts of Hb F, averaging 60%–80%. The affinity of Hb F for oxygen is greater than that of Hb A because of poor binding of 2,3-diphosphoglycerate. This results in a shift of the oxygen dissociation curve to the left, which is favorable for oxygen transport to the fetus in the relative hypoxia of intrauterine existence but which may be disadvantageous after birth [21]. The high level of Hb F at birth offers temporary protection from β hemoglobinopathies, such as sickle cell anemia, and may hamper their diagnosis in the newborn. Roland Scott, using the relatively insensitive “sickle cell prep,” demonstrated a much lower frequency of “sicklemia” in black newborns than was found in older children from the same community [22]. The development of techniques such as acid agar gel electrophoresis and high pressure liquid chromatography have permitted genotypic diagnosis of most hemoglobinopathies at birth, and neonatal testing for hemoglobinopathies is now performed routinely in all 50 states of the USA [23].
The only somewhat common hemoglobinopathy that produces symptoms in the newborn is homozygous alpha-thalassemia resulting from deletion of four alpha-globin genes [24]. In parts of Southeast Asia, fetal hydrops is caused much more frequently by alpha-thalassemia than by Rh immunization. The recent immigrations of large numbers of Southeast Asian people into the USA have resulted in increasing numbers of affected infants. Some of these have survived after intrauterine transfusions but are transfusion dependent [25].
Other inherited conditions such as hereditary spherocytosis, a defect in RBC spectrin, may be associated with non-immune hemolysis, both in utero and in the neonatal period, resulting in anemia and hyperbilirubinemia. Inherited deficiency of RBC enzymes such as glucose 6-phosphate dehydrogenase combined with exposure to oxidant substances, perhaps fava beans, causes epidemic neonatal hyperbilirubinemia in Greek infants.
Since the turn of the twentieth century, a large number of studies of the hematology and blood diseases of the newborn have been reported. Much of this information has been incorporated into textbooks of hematology. Dr. Maxwell Wintrobe’s monumental Clinical Hematology, which was first published in 1943, contained sections on normal blood values, anemias, and hemorrhagic diseases of the newborn. Neonatal thrombocytopenia in infants born of mothers with immune thrombocytopenic purpura (ITP) was also mentioned briefly. In subsequent editions of Wintrobe’s text, many more neonatal hematological conditions were described. In 1960, Dr. Carl Smith published Blood Diseases of Infancy and Childhood, the first American textbook of pediatric hematology/oncology. This had several chapters devoted to normal values and hematologic problems in the neonatal period.
In 1966, Drs. Frank Oski and Laurie Naiman published Hematological Problems in the Newborn, the first text devoted solely to the hematology and hematological problems of the neonate [26]. The authors’ stated purpose was
… to provide in a single source much of what is known concerning both the normal and abnormal hematologic processes of the first month of life and the effects of prenatal factors on them … And to provide a useful guide to all who care for the newborn infant – those who are continually confronted with infants who are bleeding, anemic or jaundiced.
The Oski–Naiman text had two subsequent editions in 1972 and 1982. Subsequently, there have been a plethora of texts and handbooks on pediatric hematology, most of which devote chapters to the newborn.
The history of neonatal hematology and the process of understanding hematologic diseases based on clinical and laboratory observations that stimulate investigation of basic mechanisms and then therapeutic interventions are illustrated well by three quintessential neonatal blood conditions: erythroblastosis fetalis; hemorrhagic disease of the newborn; and physiological anemia of infancy.
Erythroblastosis Fetalis
As recently as 1946, erythroblastosis fetalis, or hemolytic disease of the newborn, affected between 0.5% and 1.0% of fetuses and newborns in the USA. It had a 50% mortality as well as significant neurologic morbidity in many survivors [27]. Prior to 1936, three seemingly distinct neonatal syndromes had been identified: fetal hydrops, icterus gravis neonatorum, and anemia of the newborn. Based on histological and hematological similarities, Drs. L.K. Diamond, K. Blackfan, and J. Baty advanced a unifying hypothesis that these three syndromes were manifestations of a single underlying disease process. They designated all of these neonatal syndromes “erythroblastosis fetalis” because of massive nucleated RBC proliferation in the organs and blood [28].
Dr. Ruth Darrow was a pathologist who had several of her own children die of erythroblastosis. In 1938, she advanced a brilliant inductive hypothesis about its cause. Assembling all of the available information, as well as drawing on her tragic personal experiences, she noted the usual sparing of the first child and the progressive involvement of most subsequently born children. She recognized that the clinical, hematologic, and histopathologic findings in these infants could be best explained by severe hemolysis. She concluded that the disease results because [29]:
The mother is actively immunized against fetal red cells or some component of them … The antibodies formed in the maternal organism may then pass to the child through the placenta.
The elusive offending red-cell antigen and its antibody were discovered in 1940 by Drs. Karl Landsteiner and Alexander Weiner. It was given the name Rh (rhesus factor) because the antibody was produced by injection of red-blood cells of rhesus monkeys into rabbits. This antibody agglutinated the red cells of 85% of normal individuals [30]. Interestingly, Landsteiner’s discovery of the Rh blood group was accomplished almost 40 years after he had discovered the ABO blood groups [31]. In 1941, Dr. Philip Levine and associates described a severe transfusion reaction in a postpartum woman who received a transfusion of her husband’s blood shortly after delivering a stillborn baby with hydrops fetalis. Levine was able to demonstrate Rh antibodies in the mother’s circulation, defining clearly the pathophysiology of erythroblastosis fetalis [32, 33].
Effective treatment for erythroblastosis progressed slowly. The treatment of icterus gravis by “exsanguination transfusion” was first reported in 1925 by Dr. A.P. Hart at the Toronto Hospital for Sick Children [34]. With the discovery of the Rh factor, exchange transfusion evolved rapidly as a way to remove circulating antibodies, sensitized red-blood cells, and bilirubin; Drs. Harry Wallerstein and Alexander Weiner in New York and Louis K. Diamond in Boston spearheaded this treatment. Wallerstein’s method involved aspiration of the neonate’s blood from the sagittal sinus and infusion of Rh negative blood into a peripheral vein [35]. Weiner’s method employed heparinization and surgical cannulation of the radial artery and saphenous vein. Interestingly, at a time long before institutional review boards for research, he first evaluated the technique in a nonerythroblastic “Mongolian idiot” [36]. Diamond’s much more practical method utilized the umbilical vein to alternately remove and infuse blood, and this rapidly became the accepted method around the world [37]. Drs. Diamond and F. Allen developed practical guidelines for the prenatal and postnatal management of Rh-sensitized mothers and their erythroblastotic newborns. These reduced neonatal mortality from 50% to 5% and intrauterine death from 20% to less than 10%.
Kernicterus associated with severe hyperbilirubinemia was virtually eliminated [38]. Implicit in the pathogenesis of Rh erythroblastosis is that small numbers of fetal erythrocytes gain entrance into the maternal circulation, particularly during labor, where they evoke maternal immunization and Rh antibody formation. The possibility of large fetomaternal transfusion was first hypothesized by Dr. A. Weiner and proven definitively by Dr. Bruce Chown, who used differential agglutination to demonstrate and quantitate fetal red cells in the maternal circulation in a case of neonatal anemia [39, 40]. It is now recognized that acute, massive fetomaternal transfusion during labor can result in severe anemia, neonatal pallor and hypovolemic shock resembling asphyxia pallida. Chronic fetal/maternal hemorrhage during pregnancy may be associated with a well-compensated congenital microcytic hypochromic anemia due to iron deficiency because of chronic blood loss by the fetus [41].
Two penultimate important developments in erythroblastosis fetalis were provided by Dr. A.W. Liley of New Zealand, who devised a method of spectroscopic analysis of amniotic fluid to determine increase in fetal bilirubin as a result of hemolysis. This enabled identification of immunized fetuses who were at high risk of intrauterine death. Liley showed that these severely affected infants could be given intrauterine intraperitoneal transfusion of Rh negative RBC to carry them to delivery [42, 43]. Development of percutaneous umbilical blood sampling under ultrasonographic guidance has enabled perinatologists to directly diagnose and assess the severity of anemia in immunized fetuses and to treat them with simple or exchange transfusion in utero.
In 1967, Drs. C.A. Clark in Liverpool and V.J. Freda and associates in New York showed independently that primary isoimmunization of Rh-negative mothers by the Rh-positive red cells of their fetuses could be largely prevented by immediate postnatal administration of potent anti-Rh gamma globulin to the mother [44, 45]. In most of the developed world, erythroblastosis fetalis has become a rare disease of largely historical interest, and exchange transfusion has become a lost skill [46].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree