Abstract
Over the last decades, as the survival of neonates admitted to the neonatal intensive care unit (NICU) improved, thrombocytopenia became an increasingly important problem in the care of sick term and particularly preterm neonates. In this population, the majority of thrombocytopenias are due to acquired processes, and most resolve with time and/or treatment of the underlying illness. Frequently, however, the etiology of the thrombocytopenia poses a diagnostic dilemma, and – if severe enough – may place the affected neonate at risk of bleeding.
Introduction
Over the last decades, as the survival of neonates admitted to the neonatal intensive care unit (NICU) improved, thrombocytopenia became an increasingly important problem in the care of sick term and particularly preterm neonates. In this population, the majority of thrombocytopenias are due to acquired processes, and most resolve with time and/or treatment of the underlying illness. Frequently, however, the etiology of the thrombocytopenia poses a diagnostic dilemma, and – if severe enough – may place the affected neonate at risk of bleeding.
In this chapter, we review the incidence of neonatal thrombocytopenia and propose a classification and diagnostic approach based on timing and clinical presentation, as well as on novel tests of platelet production. Next, we discuss the risks and benefits associated with platelet transfusions. Acquired, immune, and congenital varieties of neonatal thrombocytopenia are discussed in detail in Chapters 13, 14, and 15, respectively.
Definition and Incidence of Neonatal Thrombocytopenia
In general, at the time of birth, the platelet count in neonates is similar to that in older children and adults, ranging from 150 to 450 × 109/L [1–3]. Aballi and colleagues reported a mean platelet count in full-term newborns of 250 × 109/L, with a range from 117 to 450 × 109/L [1]. Ablin and colleagues [2] reported a mean platelet count of 190 × 109/L, with a range from 84 to 478 × 109/L. In that study, 4% of full-term babies had a platelet count below 150 × 109/L. Sell and Corrigan [3] reported a mean platelet count in full-term newborns of 325 × 109/L, with a standard error of 50 × 109/L. Premature infants at birth, in general, also have mean platelet counts within the normal range of older children or adults, although platelet counts <150 × 109/L are more frequent among preterm than among term infants. In the series by Aballi and colleagues, 14% of preterm infants had a platelet count below 150 × 109/L [1].
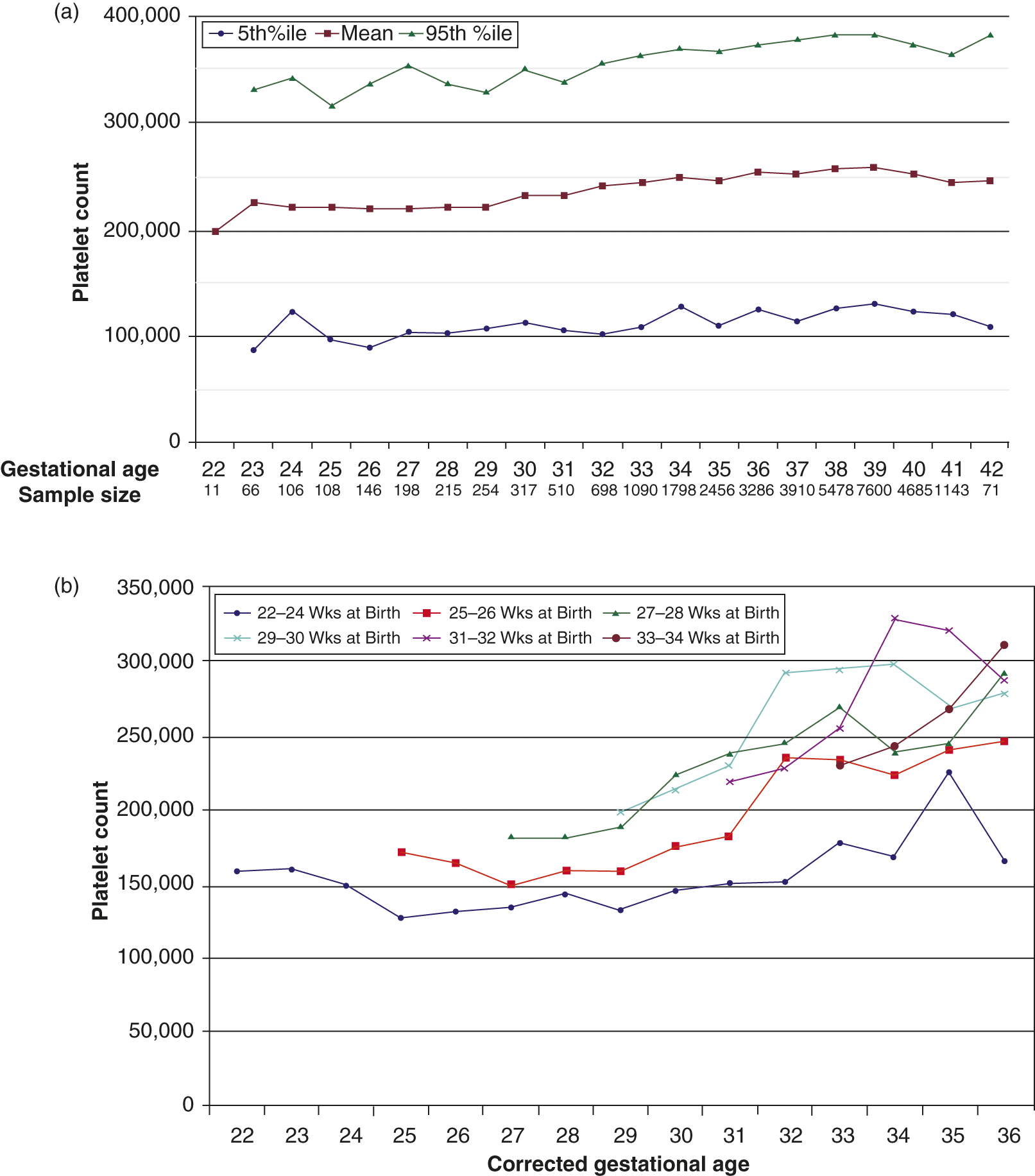
In a recent very large population study involving 47,291 neonates from a multi-hospital system, reference ranges for platelet counts at different gestational and post-conceptional ages were determined by excluding the top and lower 5th percentile of all platelet counts [4]. Using this approach, the lowest limit (5th percentile) of platelet counts for infants <32 weeks’ gestation at birth was found to be 104 × 109/L, compared to 123 × 109/L for late preterm/term neonates (>32 weeks) (Fig. 12.1a). While this is the largest study of platelet counts in neonates published to date, it is important to keep in mind that ill neonates were not excluded from this study, so that these values should be regarded as epidemiological “reference ranges” for neonates admitted to the neonatal intensive care unit (NICU), rather than as “normal ranges” for this population.
Fig. 12.1 (a) First recorded platelet counts obtained in the first 3 days after birth in neonates 22 to 42 weeks’ gestation. The red line represents the mean values and the blue and green lines represent the 5th and 95th percentiles, respectively. (b) Effects of postnatal age on mean platelet counts of premature infants. The mean platelet counts were plotted according to gestational age at birth. Data were grouped according to weeks of gestation completed at birth as follows: 22 to 24 weeks, 25 to 26 weeks, 27 to 28 weeks, 31 to 32 weeks, and 33 to 34 weeks.
An additional finding from this study was a strong correlation between the gestational age at birth and the time required postnatally before the platelet count began to increase and reached values similar to those of term infants. Specifically, the investigators noticed that the mean postnatal platelet counts of infants born at 29 to 34 weeks increased steadily over the first 2–4 weeks of life, and then stabilized at levels similar to those of term infants. In contrast, the postnatal platelet counts of infants born between 22 and 27 weeks did not begin to increase until they reached a post-conceptional age of approximately 29 weeks. Furthermore, the platelet counts of the most immature infants (born at 22–25 weeks) always remained below the mean levels measured in near-term and term infants (Fig. 12.1b). Similar findings were reported by McPherson and Juul in a study following platelet counts in infants born between 24 and 40 weeks’ gestation over the first 4 weeks of life [5]. The mechanisms underlying these observations are unknown, but it is likely that developmental differences between fetal, neonatal, and adult megakaryocytopoiesis play a role (see Chapter 13 for details).
Thrombocytopenia in neonates has traditionally been defined as a platelet count <150 × 109/L, and has been classified as mild (100–150 × 109/L), moderate (50–99 × 109/L), and severe (<50 × 109/L). Applying those definitions, large studies in unselected populations established an overall incidence of neonatal thrombocytopenia of 0.7 to 0.9% [6, 7]. In contrast, investigators focusing on neonates admitted to the NICU found a much higher incidence of thrombocytopenia, ranging from 18 to 35% [8–10]. Consistent with the observations of lower mean platelet counts in the most preterm infants, the incidence of neonatal thrombocytopenia has also been shown to be inversely related to gestational age, so that the most immature neonates are the most frequently affected. Christensen et al. conducted a study limited to extremely low birth weight neonates (ELBW, <1,000 g), and found that 73% had at least one recorded platelet count <150 × 109/L [11].
Classification and Evaluation of Neonatal Thrombocytopenia
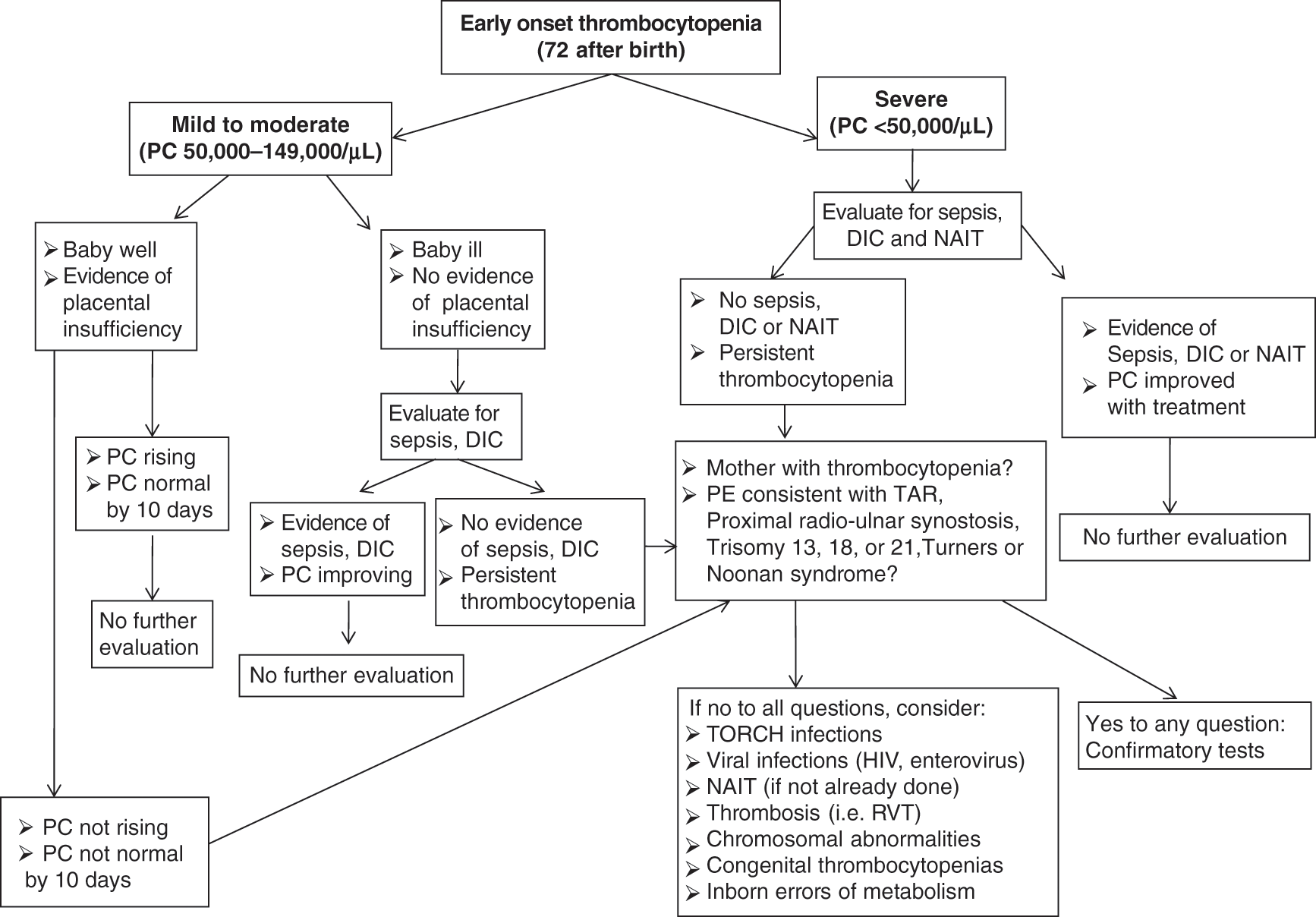
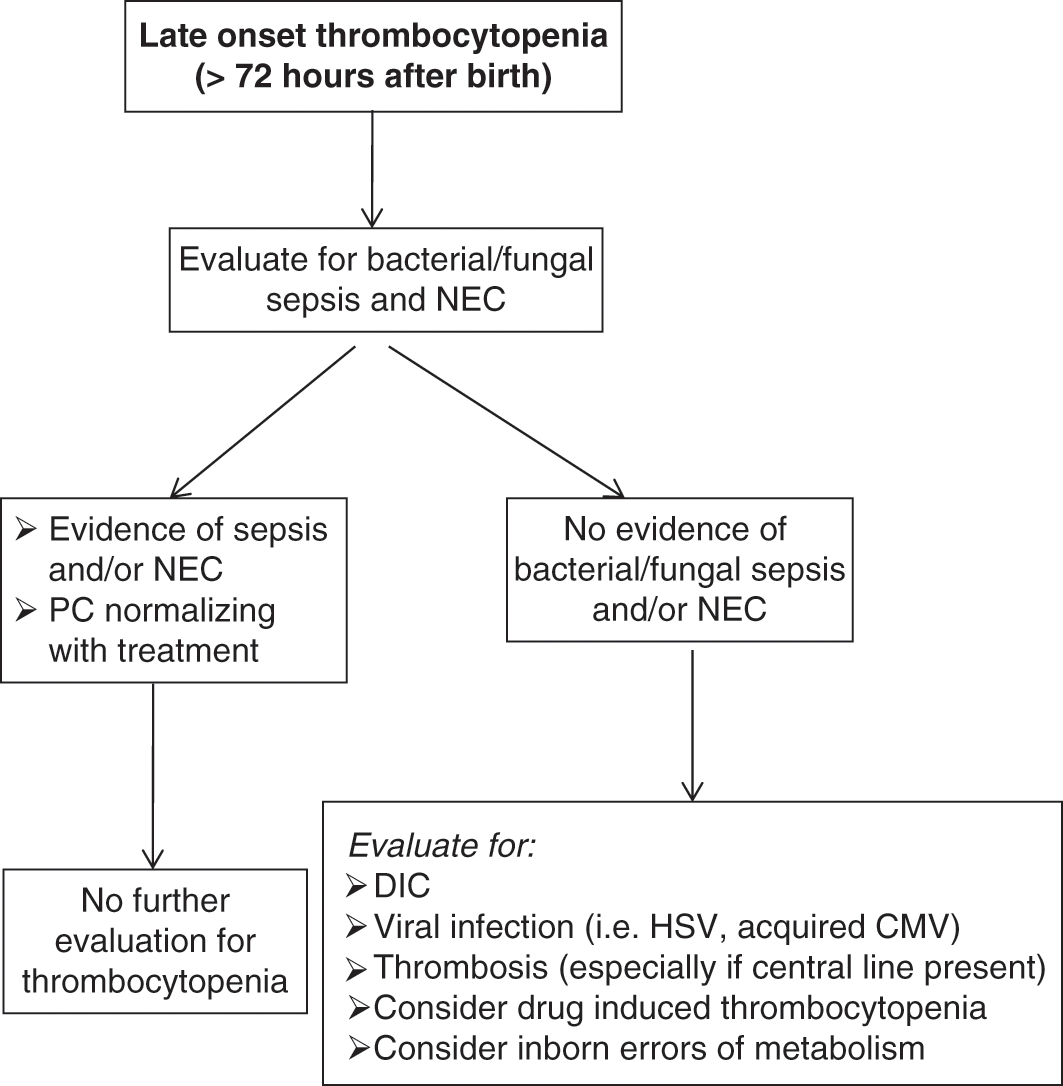
The timing of presentation of the thrombocytopenia is a helpful factor when narrowing the differential diagnosis, although there is significant overlap and several conditions can present with thrombocytopenia of early- or late-onset (i.e., sepsis or thromboses). Congenital and immune (allo- and auto-immune) thrombocytopenias usually present during the first 72 hours of life, and are reviewed in detail in Chapters 13, 14 and 15. Thrombocytopenias presenting after 72 hours (late onset) are most commonly acquired and not mediated by immune mechanisms – these will be reviewed in Chapter 13. Algorithms describing the diagnostic approach to thrombocytopenic neonates with early onset or late onset thrombocytopenia are presented in Figs. 12.2 and 12.3.
Fig. 12.2 Diagnostic approach to neonates with early-onset thrombocytopenia (presenting in the first 72 hours of life).
Fig. 12.3 Diagnostic approach to neonates with late-onset thrombocytopenia (presenting >72 hours after birth).
Early-Onset Thrombocytopenia
In neonates with early-onset thrombocytopenia (<72 hours of life), the initial evaluation is based on the severity of thrombocytopenia, the clinical presentation, and the maternal history (see diagnostic algorithm in Fig. 12.2). A documented maternal history of pregnancy-induced hypertension, chronic hypertension, and/or intrauterine growth restriction (all of which are associated with placental insufficiency and chronic intrauterine hypoxia) is particularly helpful in narrowing the differential diagnosis. Placental insufficiency is the most common etiology of transient mild thrombocytopenia in non-septic appearing neonates (particularly those born preterm) and can be managed expectantly with close observation. However, either the development of severe thrombocytopenia or the lack of resolution within 10 days should prompt evaluation for other causes. Congenital viral or parasitic infections (i.e., CMV, or toxoplasma infections) and genetic disorders, in particular, can present with thrombocytopenia and intrauterine growth restriction as the most salient features in the neonatal period, and their diagnosis requires a high index of suspicion. Close monitoring of the clinical condition is also very important, since thrombocytopenia can be the first presenting sign of a serious condition (i.e., sepsis, hemophagocytic lymphohistiocytosis). For this reason, many clinicians choose to send blood cultures and closely monitor the clinical status of well-appearing neonates in whom the etiology of the thrombocytopenia is not yet clearly defined. Severe early-onset thrombocytopenia in an otherwise healthy or mildly symptomatic infant should trigger suspicion for immune-mediated thrombocytopenia, either autoimmune (i.e., the mother is also thrombocytopenic) or alloimmune (the mother has a normal platelet count). The evaluation and management of alloimmune thrombocytopenia is reviewed in detail in Chapter 14.
Early-onset thrombocytopenia (of any severity) in an ill-appearing term or preterm neonate or in a neonate with abnormal liver enzymes and/or coagulation tests should prompt evaluation for bacterial sepsis, viral or parasitic infections, or DIC (i.e., related to perinatal asphyxia). The physical exam is also extremely important in all patients with thrombocytopenia, since it can provide important clues to the diagnosis, such as subtle congenital anomalies in genetic disorders [12], hepatosplenomegaly in congenital viral infections [13] or in hemophagocytic lymphohistiocytosis (HLH), or a palpable abdominal mass in renal vein thrombosis (RVT) [14]. Appropriate tests should be sent, and treatment should be initiated based on index of suspicion. Many of these cases require hematology consultation for appropriate diagnosis and management, and presenting features and mechanisms are reviewed in Chapter 13 and 15.
Late-Onset Thrombocytopenia
Late-onset thrombocytopenia (≥72 hours after birth) should always prompt rapid evaluation and potential treatment for bacterial/fungal sepsis, necrotizing enterocolitis, or viral infections such as HSV, acquired CMV, or enteroviruses (Fig. 12.3). If these most common etiologies have been ruled out, other potential causes include inborn errors of metabolism, thromboses (i.e., catheter-associated thrombosis), or drug-induced thrombocytopenias (see Acquired Thrombocytopenias, Chapter 13).
Measurements of Neonatal Platelet Production
In cases where the etiology of the thrombocytopenia is unclear, assessing the platelet production can be very helpful to identify the mechanism of the thrombocytopenia (decreased platelet production vs. increased consumption vs. a combination of the two), narrow the differential diagnosis and predict the course of the thrombocytopenia. In adults, bone marrow biopsy is the gold standard test for the mechanistic evaluation of thrombocytopenia. In neonates, however, this procedure is technically difficult, and is frequently postponed until the infant is larger and/or out of the neonatal period. With the hope of overcoming this limitation, a number of potentially useful indirect measurements of platelet production were developed, including plasma or serum thrombopoietin (Tpo) concentrations, circulating megakaryocyte (MK) progenitors, and reticulated platelet percentages (RP%). The circulating Tpo concentrations reflect the balance between Tpo production and Tpo binding to available receptors [15]. Thus, elevated levels are seen in cases of up-regulated Tpo production, such as during infection, or in cases of bone marrow suppression and decreased megakaryocytes, such as in congenital amegakaryocytic thrombocytopenia or infiltrative bone marrow diseases [16]. Circulating MK progenitors have been used as a reflection of bone marrow MKs [17, 18], although no studies to date have directly correlated these measures in neonates. Reticulated platelets are platelets recently released from the bone marrow (<24 hours old), which can be identified by their high RNA content [19]. In a manner similar to reticulocytes in anemia, the reticulated platelet percentage is elevated in consumptive thrombocytopenias, and is low in thrombocytopenias due to decreased platelet production [20]. Four studies evaluated the RP% in fetuses and neonates, with three of the four demonstrating similar to higher RP% in neonates compared to adults [21–24].
Until recently, all of these tests were only available in the research setting. However, a test similar to the RP% was recently developed for clinical use. This RP% equivalent, termed the immature platelet fraction (IPF) can be measured in Sysmex standard hematology analyzers (Sysmex, Kobe, Japan) as part of a routine complete blood count. Similarly to the RP%, the IPF% is elevated in thrombocytopenic conditions associated with increased platelet destruction (e.g., immune thrombocytopenic purpura, ITP), and decreased in thrombocytopenias due to decreased platelet production (e.g., aplastic anemia) [25]. Only a few studies so far have evaluated IPF values in neonates. Cremer and collaborators were the first to report the IPF in non-thrombocytopenic infants and in neonates with early-onset thrombocytopenia [26]. Based on their findings, these investigators suggested that the IPF could be used to predict recovery of the platelet count within the next 24 hours [26]. Ko and collaborators generated IPF reference ranges for full term neonates using cord blood samples and found that they were similar to adult reference ranges [27]. More recently, two groups examined the IPF in preterm neonates [28, 29]. In the largest IPF study published to date, MacQueen et al. examined 24,372 platelet counts and IPF percentages from 9,172 term and preterm neonates 0–90 days old [29]. Data from non-thrombocytopenic infants in this cohort were used to generate age-specific reference intervals for IPF% and immature platelet counts (IPC, calculated as IPF% × platelet count) (Fig. 12.4) [29]. As seen in the figure, the IPF at the time of birth was higher in preterm infants, and decreased trough gestation until 32 weeks, at which time the IPF stabilized at full-term values. Postnatally, the IPF increased progressively over the first 2 weeks of life and returned to baseline by 1 month in all gestational ages. Importantly, this study also assessed IPF percentages in neonates with thrombocytopenia, and found significantly higher values in neonates with consumptive etiologies compared to those with thrombocytopenia secondary to decreased production (Table 12.1). Thus, when available, the IPF can be a useful clinical tool in neonates, in whom it may help discern the underlying mechanisms of thrombocytopenia, narrow the differential diagnosis, and predict platelet recovery.
Table 12.1 Immature platelet fraction values in neonates with thrombocytopenia of different etiologies
| Mechanism of thrombocytopenia | N | IPF% |
|---|---|---|
| Hypoproliferative* | 92 | 10.4 ± 2.9 |
| Consumptive** | 98 | 20.9 ± 7.9 |
| Both | 76 | 17.9 ± 5.9 |
| Indeterminate*** | 14 | 12.8 ± 8.1 |
Notes: *SGA, birth asphyxia or a syndrome associated with hypoproliferative thrombocytopenia;
**Immune-mediated, NEC, sepsis, or DIC;
***None of the above diagnoses.
(Adapted from: MacQueen et al., J Perinatol 37, 2017 [29].)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree